The ellagitannin metabolite urolithin C is a glucose-dependent regulator of insulin secretion through activation of L-type calcium channels
- PMID: 31378934
- PMCID: PMC6811744
- DOI: 10.1111/bph.14821
The ellagitannin metabolite urolithin C is a glucose-dependent regulator of insulin secretion through activation of L-type calcium channels
Abstract
Background and purpose: The pharmacology of polyphenol metabolites on beta-cell function is largely undetermined. We sought to identify polyphenol metabolites that enhance the insulin-secreting function of beta-cells and to explore the underlying mechanisms.
Experimental approach: INS-1 beta-cells and rat isolated islets of Langerhans or perfused pancreas preparations were used for insulin secretion experiments. Molecular modelling, intracellular Ca2+ monitoring, and whole-cell patch-clamp recordings were used for mechanistic studies.
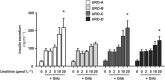
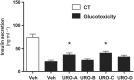
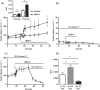
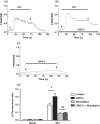
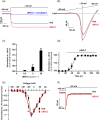
Key results: Among a set of polyphenol metabolites, we found that exposure of INS-1 beta-cells to urolithins A and C enhanced glucose-stimulated insulin secretion. We further characterized the activity of urolithin C and its pharmacological mechanism. Urolithin C glucose-dependently enhanced insulin secretion in isolated islets of Langerhans and perfused pancreas preparations. In the latter, enhancement was reversible when glucose was lowered from a stimulating to a non-stimulating concentration. Molecular modelling suggested that urolithin C could dock into the Cav 1.2 L-type Ca2+ channel. Calcium monitoring indicated that urolithin C had no effect on basal intracellular Ca2+ but enhanced depolarization-induced increase in intracellular Ca2+ in INS-1 cells and dispersed cells isolated from islets. Electrophysiology studies indicated that urolithin C dose-dependently enhanced the L-type Ca2+ current for levels of depolarization above threshold and shifted its voltage-dependent activation towards more negative potentials in INS-1 cells.
Conclusion and implications: Urolithin C is a glucose-dependent activator of insulin secretion acting by facilitating L-type Ca2+ channel opening and Ca2+ influx into pancreatic beta-cells. Our work paves the way for the design of polyphenol metabolite-inspired compounds aimed at ameliorating beta-cell function.
Background and Purpose: The pharmacology of polyphenol metabolites on beta‐cell function is largely undetermined. We sought to identify polyphenol metabolites that enhance the insulin‐secreting function of beta‐cells and to explore the underlying mechanisms.
Experimental Approach: INS‐1 beta‐cells and rat isolated islets of Langerhans or perfused pancreas preparations were used for insulin secretion experiments. Molecular modelling, intracellular Ca2+ monitoring, and whole‐cell patch‐clamp recordings were used for mechanistic studies.
Key Results: Among a set of polyphenol metabolites, we found that exposure of INS‐1 beta‐cells to urolithins A and C enhanced glucose‐stimulated insulin secretion. We further characterized the activity of urolithin C and its pharmacological mechanism. Urolithin C glucose‐dependently enhanced insulin secretion in isolated islets of Langerhans and perfused pancreas preparations. In the latter, enhancement was reversible when glucose was lowered from a stimulating to a non‐stimulating concentration. Molecular modelling suggested that urolithin C could dock into the Cav1.2 L‐type Ca2+ channel. Calcium monitoring indicated that urolithin C had no effect on basal intracellular Ca2+ but enhanced depolarization‐induced increase in intracellular Ca2+ in INS‐1 cells and dispersed cells isolated from islets. Electrophysiology studies indicated that urolithin C dose‐dependently enhanced the L‐type Ca2+ current for levels of depolarization above threshold and shifted its voltage‐dependent activation towards more negative potentials in INS‐1 cells.
Conclusion and Implications: Urolithin C is a glucose‐dependent activator of insulin secretion acting by facilitating L‐type Ca2+ channel opening and Ca2+ influx into pancreatic beta‐cells. Our work paves the way for the design of polyphenol metabolite‐inspired compounds aimed at ameliorating beta‐cell function.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Alexander, S. P. H. , Striessnig, J. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Voltage‐gated ion channels: THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Voltage‐gated ion channels. British Journal of Pharmacology, 174, S160–S194. 10.1111/bph.13884 - DOI - PMC - PubMed
-
- Bardy, G. , Virsolvy, A. , Quignard, J. F. , Ravier, M. A. , Bertrand, G. , Dalle, S. , … Oiry, C. (2013). Quercetin induces insulin secretion by direct activation of L‐type calcium channels in pancreatic beta cells. British Journal of Pharmacology, 169, 1102–1113. 10.1111/bph.12194 - DOI - PMC - PubMed
-
- Bayle, M. , Roques, C. , Marion, B. , Audran, M. , Oiry, C. , Bressolle‐Gomeni, F. M. M. , & Cros, G. (2016). Development and validation of a liquid chromatography‐electrospray ionization‐tandem mass spectrometry method for the determination of urolithin C in rat plasma and its application to a pharmacokinetic study. Journal of Pharmaceutical and Biomedical Analysis, 131, 33–39. 10.1016/j.jpba.2016.07.046 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

