Enriched Environment Shortens the Duration of Action Potentials in Cerebellar Granule Cells
- PMID: 31379501
- PMCID: PMC6646744
- DOI: 10.3389/fncel.2019.00289
Enriched Environment Shortens the Duration of Action Potentials in Cerebellar Granule Cells
Abstract
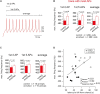
Environmental enrichment for rodents is known to enhance motor performance. Structural and molecular changes have been reported to be coupled with an enriched environment, but functional alterations of single neurons remain elusive. Here, we compared mice raised under control conditions and an enriched environment. We tested the motor performance on a rotarod and subsequently performed whole-cell patch-clamp recordings in cerebellar slices focusing on granule cells of lobule IX, which is known to receive vestibular input. Mice raised in an enriched environment were able to remain on an accelerating rotarod for a longer period of time. Electrophysiological analyses revealed normal passive properties of granule cells and a functional adaptation to the enriched environment, manifested in faster action potentials (APs) with a higher depolarized voltage threshold and larger AP overshoot. Furthermore, the maximal firing frequency of APs was higher in mice raised in an enriched environment. These data show that enriched environment causes specific alterations in the biophysical properties of neurons. Furthermore, we speculate that the ability of cerebellar granule cells to generate higher firing frequencies improves motor performance.
Keywords: action potential; cerebellum; electrophysiology; enriched environment; granule cell.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

