Alzheimer's Disease as a Membrane Disorder: Spatial Cross-Talk Among Beta-Amyloid Peptides, Nicotinic Acetylcholine Receptors and Lipid Rafts
- PMID: 31379503
- PMCID: PMC6657435
- DOI: 10.3389/fncel.2019.00309
Alzheimer's Disease as a Membrane Disorder: Spatial Cross-Talk Among Beta-Amyloid Peptides, Nicotinic Acetylcholine Receptors and Lipid Rafts
Abstract
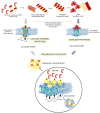
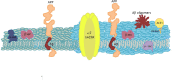
Biological membranes show lateral and transverse asymmetric lipid distribution. Cholesterol (Chol) localizes in both hemilayers, but in the external one it is mostly condensed in lipid-ordered microdomains (raft domains), together with saturated phosphatidyl lipids and sphingolipids (including sphingomyelin and glycosphingolipids). Membrane asymmetries induce special membrane biophysical properties and behave as signals for several physiological and/or pathological processes. Alzheimer's disease (AD) is associated with a perturbation in different membrane properties. Amyloid-β (Aβ) plaques and neurofibrillary tangles of tau protein together with neuroinflammation and neurodegeneration are the most characteristic cellular changes observed in this disease. The extracellular presence of Aβ peptides forming senile plaques, together with soluble oligomeric species of Aβ, are considered the major cause of the synaptic dysfunction of AD. The association between Aβ peptide and membrane lipids has been extensively studied. It has been postulated that Chol content and Chol distribution condition Aβ production and posterior accumulation in membranes and, hence, cell dysfunction. Several lines of evidence suggest that Aβ partitions in the cell membrane accumulate mostly in raft domains, the site where the cleavage of the precursor AβPP by β- and γ- secretase is also thought to occur. The main consequence of the pathogenesis of AD is the disruption of the cholinergic pathways in the cerebral cortex and in the basal forebrain. In parallel, the nicotinic acetylcholine receptor has been extensively linked to membrane properties. Since its transmembrane domain exhibits extensive contacts with the surrounding lipids, the acetylcholine receptor function is conditioned by its lipid microenvironment. The nicotinic acetylcholine receptor is present in high-density clusters in the cell membrane where it localizes mainly in lipid-ordered domains. Perturbations of sphingomyelin or cholesterol composition alter acetylcholine receptor location. Therefore, Aβ processing, Aβ partitioning, and acetylcholine receptor location and function can be manipulated by changes in membrane lipid biophysics. Understanding these mechanisms should provide insights into new therapeutic strategies for prevention and/or treatment of AD. Here, we discuss the implications of lipid-protein interactions at the cell membrane level in AD.
Keywords: Alzheimer’s disease; Aβ peptide; acetylcholinesterase; cell membranes; cholesterol; lipid rafts; nicotinic acetylcholine receptor.
Figures

References
-
- Ahyayauch H., Raab M., Busto J. V., Andraka N., Arrondo J. L., Masserini M., et al. (2012). Binding of β-amyloid (1-42) peptide to negatively charged phospholipid membranes in the liquid-ordered state: modeling and experimental studies. Biophys. J. 103 453–463. 10.1016/j.bpj.2012.06.043 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

