Dorsal Striatal Circuits for Habits, Compulsions and Addictions
- PMID: 31379523
- PMCID: PMC6657020
- DOI: 10.3389/fnsys.2019.00028
Dorsal Striatal Circuits for Habits, Compulsions and Addictions
Abstract
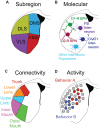
Here, we review the neural circuit bases of habits, compulsions, and addictions, behaviors which are all characterized by relatively automatic action performance. We discuss relevant studies, primarily from the rodent literature, and describe how major headway has been made in identifying the brain regions and neural cell types whose activity is modulated during the acquisition and performance of these automated behaviors. The dorsal striatum and cortical inputs to this structure have emerged as key players in the wider basal ganglia circuitry encoding behavioral automaticity, and changes in the activity of different neuronal cell-types in these brain regions have been shown to co-occur with the formation of automatic behaviors. We highlight how disordered functioning of these neural circuits can result in neuropsychiatric disorders, such as obsessive-compulsive disorder (OCD) and drug addiction. Finally, we discuss how the next phase of research in the field may benefit from integration of approaches for access to cells based on their genetic makeup, activity, connectivity and precise anatomical location.
Keywords: dorsolateral striatum; dorsomedial striatum; goal-directed behavior; habits; prefrontal cortex; striatum.
Figures

References
-
- Amaya K. A., Smith K. S. (2018). Neurobiology of habit formation. Curr. Opin. Behav. Sci. 20, 145–152. 10.1016/j.cobeha.2018.01.003 - DOI
Publication types
LinkOut - more resources
Full Text Sources

