The Mechanisms of Disease Caused by Acinetobacter baumannii
- PMID: 31379771
- PMCID: PMC6650576
- DOI: 10.3389/fmicb.2019.01601
The Mechanisms of Disease Caused by Acinetobacter baumannii
Abstract
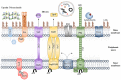
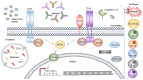
Acinetobacter baumannii is a Gram negative opportunistic pathogen that has demonstrated a significant insurgence in the prevalence of infections over recent decades. With only a limited number of "traditional" virulence factors, the mechanisms underlying the success of this pathogen remain of great interest. Major advances have been made in the tools, reagents, and models to study A. baumannii pathogenesis, and this has resulted in a substantial increase in knowledge. This article provides a comprehensive review of the bacterial virulence factors, the host immune responses, and animal models applicable for the study of this important human pathogen. Collating the most recent evidence characterizing bacterial virulence factors, their cellular targets and genetic regulation, we have encompassed numerous aspects important to the success of this pathogen, including membrane proteins and cell surface adaptations promoting immune evasion, mechanisms for nutrient acquisition and community interactions. The role of innate and adaptive immune responses is reviewed and areas of paucity in our understanding are highlighted. Finally, with the vast expansion of available animal models over recent years, we have evaluated those suitable for use in the study of Acinetobacter disease, discussing their advantages and limitations.
Keywords: A. baumannii; animal models; bacterial virulence factors; host-pathogen interactions; immune response.
Figures
References
-
- Ahmad T. A., Tawfik D. M., Sheweita S. A., Haroun M., El-Sayed L. H. (2016). Development of immunization trials against Acinetobacter baumannii. Trials Vaccinol. 5, 53–60. 10.1016/j.trivac.2016.03.001 - DOI
-
- Ainsworth S., Ketter P. M., Yu J.-J., Grimm R. C., May H. C., Cap A. P., et al. (2017). Vaccination with a live attenuated Acinetobacter baumannii deficient in thioredoxin provides protection against systemic Acinetobacter infection. Vaccine 35, 3387–3394. 10.1016/j.vaccine.2017.05.017, PMID: - DOI - PMC - PubMed
-
- Álvarez-Fraga L., Vázquez-Ucha J. C., Martínez-Guitián M., Vallejo J. A., Bou G., Beceiro A., et al. (2018). Pneumonia infection in mice reveals the involvement of the feoA gene in the pathogenesis of Acinetobacter baumannii. Virulence 9, 496–509. 10.1080/21505594.2017.1420451, PMID: - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

