Daurinol Attenuates Autoimmune Arthritis via Stabilization of Nrp1-PTEN-Foxp3 Signaling in Regulatory T Cells
- PMID: 31379809
- PMCID: PMC6651269
- DOI: 10.3389/fimmu.2019.01526
Daurinol Attenuates Autoimmune Arthritis via Stabilization of Nrp1-PTEN-Foxp3 Signaling in Regulatory T Cells
Abstract
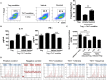
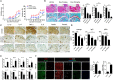
Optimizing Treg function and improving Treg stability are attractive treatment strategies for treating autoimmune rheumatoid arthritis (RA). However, the limited number of circulating Tregs and questions about the functional stability of in vitro-expanded Tregs are potential limitations of Treg-based cell therapy. The aim of this study was to analyze the regulatory effect of daurinol, a catalytic inhibitor of topoisomerase IIα, on Th cell differentiation and to evaluate their therapeutic potential in a preclinical experimental model of RA. We investigated the effect of daurinol on T cell differentiation by flow cytometry. Foxp3 stability and methylation were analyzed by suppression assays and bisulfite pyrosequencing. Daurinol was treated in the collagen-induced arthritis (CIA) model, and the effects in vivo were determined. We found that daurinol can promote Treg differentiation and reciprocally inhibit Th17 differentiation. This Treg-inducing property of daurinol was associated with decreased activity of Akt-mTOR and reciprocally increased activity of neuropilin-1 (Nrp1)-PTEN. Daurinol treatment inhibited aerobic glycolysis in Th17 conditions, indicating the metabolic changes by daurinol. We found that the daurinol increase the Treg stability was achieved by Foxp3 hypomethylation. In vivo daurinol treatment in CIA mice reduced the clinical arthritis severity and histological inflammation. The Treg population frequency increased and the Th17 cells decreased in the spleens of arthritis mice treated with daurinol. These results showed the anti-arthritic and immunoregulating properties of daurinol is achieved by increased differentiation and stabilization of Tregs. Our study provides first evidence for daurinol as a treatment for RA.
Keywords: FOXP3 hypomethylation; daurinol; neuropilin 1; regulatory T cells; rheumatoid arthritis; stability.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

