Purinergic Signaling in Pulmonary Inflammation
- PMID: 31379836
- PMCID: PMC6646739
- DOI: 10.3389/fimmu.2019.01633
Purinergic Signaling in Pulmonary Inflammation
Abstract
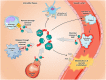
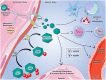
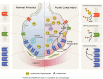
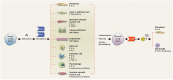
Purine nucleotides and nucleosides are at the center of biologic reactions. In particular, adenosine triphosphate (ATP) is the fundamental energy currency of cellular activity and adenosine has been demonstrated to play essential roles in human physiology and pathophysiology. In this review, we examine the role of purinergic signaling in acute and chronic pulmonary inflammation, with emphasis on ATP and adenosine. ATP is released into extracellular space in response to cellular injury and necrosis. It is then metabolized to adenosine monophosphate (AMP) via ectonucleoside triphosphate diphosphohydrolase-1 (CD39) and further hydrolyzed to adenosine via ecto-5'-nucleotidase (CD73). Adenosine signals via one of four adenosine receptors to exert pro- or anti-inflammatory effects. Adenosine signaling is terminated by intracellular transport by concentrative or equilibrative nucleoside transporters (CNTs and ENTs), deamination to inosine by adenosine deaminase (ADA), or phosphorylation back into AMP via adenosine kinase (AK). Pulmonary inflammatory and hypoxic conditions lead to increased extracellular ATP, adenosine diphosphate (ADP) and adenosine levels, which translates to increased adenosine signaling. Adenosine signaling is central to the pulmonary injury response, leading to various effects on inflammation, repair and remodeling processes that are either tissue-protective or tissue destructive. In the acute setting, particularly through activation of adenosine 2A and 2B receptors, adenosine signaling serves an anti-inflammatory, tissue-protective role. However, excessive adenosine signaling in the chronic setting promotes pro-inflammatory, tissue destructive effects in chronic pulmonary inflammation.
Keywords: acute pulmonary inflammation; adenosine; chronic pulmonary inflammation; ectonucleotidase; nucleotides; purinergic signaling.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

