The Ontogeny of Monocyte Subsets
- PMID: 31379841
- PMCID: PMC6650567
- DOI: 10.3389/fimmu.2019.01642
The Ontogeny of Monocyte Subsets
Abstract
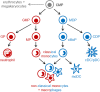
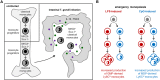
Classical and non-classical monocytes, and the macrophages and monocyte-derived dendritic cells they produce, play key roles in host defense against pathogens, immune regulation, tissue repair and many other processes throughout the body. Recent studies have revealed previously unappreciated heterogeneity among monocytes that may explain this functional diversity, but our understanding of mechanisms controlling the functional programming of distinct monocyte subsets remains incomplete. Resolving monocyte heterogeneity and understanding how their functional identity is determined holds great promise for therapeutic immune modulation. In this review, we examine how monocyte origins and developmental influences shape the phenotypic and functional characteristics of monocyte subsets during homeostasis and in the context of infection, inflammation, and cancer. We consider how extrinsic signals and transcriptional regulators impact monocyte production and functional programming, as well as the influence of epigenetic and metabolic mechanisms. We also examine the evidence that functionally distinct monocyte subsets are produced via different developmental pathways during homeostasis and that inflammatory stimuli differentially target progenitors during an emergency response. We highlight the need for a more comprehensive understanding of the relationship between monocyte ontogeny and heterogeneity, including multiparametric single-cell profiling and functional analyses. Studies defining mechanisms of monocyte subset production and maintenance of unique monocyte identities have the potential to facilitate the design of therapeutic interventions to target specific monocyte subsets in a variety of disease contexts, including infectious and inflammatory diseases, cancer, and aging.
Keywords: bone marrow; monocyte ontogeny; monocyte progenitors; monocyte subsets; monopoiesis.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

