Cytotoxic Chemotherapy as an Immune Stimulus: A Molecular Perspective on Turning Up the Immunological Heat on Cancer
- PMID: 31379850
- PMCID: PMC6652267
- DOI: 10.3389/fimmu.2019.01654
Cytotoxic Chemotherapy as an Immune Stimulus: A Molecular Perspective on Turning Up the Immunological Heat on Cancer
Abstract
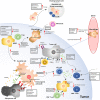
Cytotoxic chemotherapeutics (CCTs) are widely used in the treatment of cancer. Although their mechanisms of action have been best understood in terms of targeting the apparatus of mitosis, an ability to stimulate anti-tumor immune responses is increasing the recognition of these agents as immunotherapies. Immune checkpoint blockade antibodies neutralize important, but specific, immune-regulatory interactions such as PD-1/PD-L1 and CTLA-4 to improve the anti-tumor immune response. However, CCTs can provide a broad-acting immune-stimulus against cancer, promoting both T-cell priming and recruitment to the tumor, which compliments the effects of immune checkpoint blockade. A key pathway in this process is "immunogenic cell death" (ICD) which occurs as a result of tumor cell endoplasmic reticulum stress and apoptosis elicited by CCTs. ICD involves a series of non-redundant signaling events which break tolerance and license anti-tumor antigen-specific T-cells, allowing CCTs to act as "in situ" tumor vaccination tools. Not all responses are tumor cell-intrinsic, as CCTs can also modulate the broader tumor microenvironment. This modulation occurs through preferential depletion of stromal cells which suppress and neutralize robust anti-tumor immune responses, such as myeloid cell populations and Tregs, while effector CD8+ and CD4+ T-cells and NK cells are relatively spared. The immune-stimulating effects of CCTs are dependent on chemotherapy class, dose and tumor cell sensitivity to the agent, highlighting the need to understand the underlying biology of these responses. This mini review considers the immune-stimulating effects of CCTs from a molecular perspective, specifically highlighting considerations for their utilization in the context of combinations with immunotherapy.
Keywords: chemo-immunotherapy; chemotherapy; immunotherapy; microenvironment; tumor.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

