Osteoimmunology of Oral and Maxillofacial Diseases: Translational Applications Based on Biological Mechanisms
- PMID: 31379856
- PMCID: PMC6657671
- DOI: 10.3389/fimmu.2019.01664
Osteoimmunology of Oral and Maxillofacial Diseases: Translational Applications Based on Biological Mechanisms
Abstract
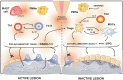
The maxillofacial skeleton is highly dynamic and requires a constant equilibrium between the bone resorption and bone formation. The field of osteoimmunology explores the interactions between bone metabolism and the immune response, providing a context to study the complex cellular and molecular networks involved in oro-maxillofacial osteolytic diseases. In this review, we present a framework for understanding the potential mechanisms underlying the immuno-pathobiology in etiologically-diverse diseases that affect the oral and maxillofacial region and share bone destruction as their common clinical outcome. These otherwise different pathologies share similar inflammatory pathways mediated by central cellular players, such as macrophages, T and B cells, that promote the differentiation and activation of osteoclasts, ineffective or insufficient bone apposition by osteoblasts, and the continuous production of osteoclastogenic signals by immune and local stromal cells. We also present the potential translational applications of this knowledge based on the biological mechanisms involved in the inflammation-induced bone destruction. Such applications can be the development of immune-based therapies that promote bone healing/regeneration, the identification of host-derived inflammatory/collagenolytic biomarkers as diagnostics tools, the assessment of links between oral and systemic diseases; and the characterization of genetic polymorphisms in immune or bone-related genes that will help diagnosis of susceptible individuals.
Keywords: biomarkers; maxillofacial; oral; osteoimmunology; periodontal disease.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

