GR Dimerization and the Impact of GR Dimerization on GR Protein Stability and Half-Life
- PMID: 31379877
- PMCID: PMC6653659
- DOI: 10.3389/fimmu.2019.01693
GR Dimerization and the Impact of GR Dimerization on GR Protein Stability and Half-Life
Abstract
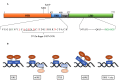
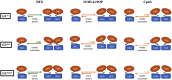
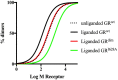
Pharmacologically, glucocorticoids, which mediate their effects via the glucocorticoid receptor (GR), are a most effective therapy for inflammatory diseases despite the fact that chronic use causes side-effects and acquired GC resistance. The design of drugs with fewer side-effects and less potential for the development of resistance is therefore considered crucial for improved therapy. Dimerization of the GR is an integral step in glucocorticoid signaling and has been identified as a possible molecular site to target for drug development of anti-inflammatory drugs with an improved therapeutic index. Most of the current understanding regarding the role of GR dimerization in GC signaling derives for dimerization deficient mutants, although the role of ligands biased toward monomerization has also been described. Even though designing for loss of dimerization has mostly been applied for reduction of side-effect profile, designing for loss of dimerization may also be a fruitful strategy for the development of GC drugs with less potential to develop GC resistance. GC-induced resistance affects up to 30% of users and is due to a reduction in the GR functional pool. Several molecular mechanisms of GC-mediated reductions in GR pool have been described, one of which is the autologous down-regulation of GR density by the ubiquitin-proteasome-system (UPS). Loss of GR dimerization prevents autologous down-regulation of the receptor through modulation of interactions with components of the UPS and post-translational modifications (PTMs), such as phosphorylation, which prime the GR for degradation. Rational design of conformationally biased ligands that select for a monomeric GR conformation, which increases GC sensitivity through improving GR protein stability and increasing half-life, may be a productive avenue to explore. However, potential drawbacks to this approach should be considered as well as the advantages and disadvantages in chronic vs. acute treatment regimes.
Keywords: Compound A; GRdim mutant; GRmon mutant; acquired glucocorticoid resistance; biased ligands; glucocorticoid receptor dimerization; half-life; ubiquitin proteasomal system.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

