T Cell Dysfunction in Cancer Immunity and Immunotherapy
- PMID: 31379886
- PMCID: PMC6659036
- DOI: 10.3389/fimmu.2019.01719
T Cell Dysfunction in Cancer Immunity and Immunotherapy
Abstract
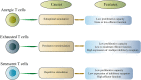
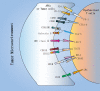
In cancer, T cells become dysfunctional owing to persistent antigen exposure. Dysfunctional T cells are characterized by reduced proliferative capacity, decreased effector function, and overexpression of multiple inhibitory receptors. Due to the presence of various inhibitory signals in the complex tumor microenvironment, tumor-specific T cells have distinct dysfunction states. Therapeutic reactivation of tumor-specific T cells has yielded good results in cancer patients. Here, we review the hallmarks of T cell dysfunction in cancer. Also, we discuss the relationship between T cell dysfunction and cancer immunotherapy.
Keywords: T cell dysfunction; cancer; immunity; immunotherapy; tumor microenvironment.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

