Notch Signaling Activation as a Hallmark for Triple-Negative Breast Cancer Subtype
- PMID: 31379945
- PMCID: PMC6657611
- DOI: 10.1155/2019/8707053
Notch Signaling Activation as a Hallmark for Triple-Negative Breast Cancer Subtype
Abstract
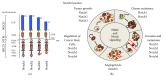
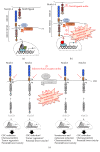
Triple-negative breast cancer (TNBC) is a subgroup of 15%-20% of diagnosed breast cancer patients. It is generally considered to be the most difficult breast cancer subtype to deal with, due to the lack of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), which usually direct targeted therapies. In this scenario, the current treatments of TNBC-affected patients rely on tumor excision and conventional chemotherapy. As a result, the prognosis is overall poor. Thus, the identification and characterization of targets for novel therapies are urgently required. The Notch signaling pathway has emerged to act in the pathogenesis and tumor progression of TNBCs. Firstly, Notch receptors are associated with the regulation of tumor-initiating cells (TICs) behavior, as well as with the aetiology of TNBCs. Secondly, there is a strong evidence that Notch pathway is a relevant player in mammary cancer stem cells maintenance and expansion. Finally, Notch receptors expression and activation strongly correlate with the aggressive clinicopathological and biological phenotypes of breast cancer (e.g., invasiveness and chemoresistance), which are relevant characteristics of TNBC subtype. The purpose of this up-to-date review is to provide a detailed overview of the specific role of all four Notch receptors (Notch1, Notch2, Notch3, and Notch4) in TNBCs, thus identifying the Notch signaling pathway deregulation/activation as a pathognomonic feature of this breast cancer subtype. Furthermore, this review will also discuss recent information associated with different therapeutic options related to the four Notch receptors, which may be useful to evaluate prognostic or predictive indicators as well as to develop new therapies aimed at improving the clinical outcome of TNBC patients.
Figures
References
-
- Torre L. A., Siegel R. L., Ward E. M., Jemal A. Global cancer incidence and mortality rates and trends--an update. Cancer Epidemiology, Biomarkers & Prevention. 2016;25(1):16–27. - PubMed
-
- Carlson R. W., Allred D. C., Anderson B. O., Burstein H. J., Carter W. B., Edge S. B., et al. Breast cancer. Clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network. 2009;7(2):122–192. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

