Lipid Profile and Aquaporin Expression under Oxidative Stress in Breast Cancer Cells of Different Malignancies
- PMID: 31379986
- PMCID: PMC6657669
- DOI: 10.1155/2019/2061830
Lipid Profile and Aquaporin Expression under Oxidative Stress in Breast Cancer Cells of Different Malignancies
Abstract
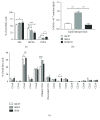
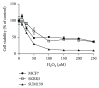
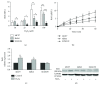
Breast cancer is the major cause of tumor-associated mortality in women worldwide, with prognosis depending on the early discovery of the disease and on the type of breast cancer diagnosed. Among many factors, lipids could contribute to breast cancer malignancy by participating in cellular processes. Also, aquaporins are membrane channels found aberrantly expressed in cancer tissues that were correlated with tumor aggressiveness, progression, and metastasis. However, the differences in lipid profile and aquaporin expression between cell types of different malignant potential have never been investigated. Here, we selected three breast cancer cell lines representing the three major breast cancer types (hormone positive, HER2NEU positive, and triple negative) and analyzed their lipid profile and steady state lipid hydroperoxide levels to correlate with cell sensitivity to H2O2. Additionally, the expression profiles of AQP1, AQP3, and AQP5 and the Nrf2 transcription factor were evaluated, before and after oxidative challenge. We found that the lipid profile was dependent on the cell type, with the HER2-positive cells having the lowest level PUFA, whereas the triple negative showed the highest. However, in triple-negative cancer cells, a lower level of the Nrf2 may be responsible for a higher sensitivity to H2O2 challenge. Interestingly, HER2-positive cells showed the highest increase in intracellular ROS after oxidative challenge, concomitant with a significantly higher level of AQP1, AQP3, and AQP5 expression compared to the other cell types, with AQP3 always being the most expressed isoform. The AQP3 gene expression was stimulated by H2O2 treatment in hormone-positive and HER2NEU cells, together with Nrf2 expression, but was downregulated in triple-negative cells that showed instead upregulation of AQP1 and AQP5. The lipid profile and AQP gene expression after oxidative challenge of these particularly aggressive cell types may represent metabolic reprogramming of cancer cells and reflect a role in adaptation to stress and therapy resistance.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

