Pulverization-Tolerance and Capacity Recovery of Copper Sulfide for High-Performance Sodium Storage
- PMID: 31380167
- PMCID: PMC6662052
- DOI: 10.1002/advs.201900264
Pulverization-Tolerance and Capacity Recovery of Copper Sulfide for High-Performance Sodium Storage
Abstract
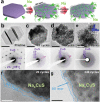
Finding suitable electrode materials is one of the challenges for the commercialization of a sodium ion battery due to its pulverization accompanied by high volume expansion upon sodiation. Here, copper sulfide is suggested as a superior electrode material with high capacity, high rate, and long-term cyclability owing to its unique conversion reaction mechanism that is pulverization-tolerant and thus induces the capacity recovery. Such a desirable consequence comes from the combined effect among formation of stable grain boundaries, semi-coherent boundaries, and solid-electrolyte interphase layers. The characteristics enable high cyclic stability of a copper sulfide electrode without any need of size and morphological optimization. This work provides a key finding on high-performance conversion reaction based electrode materials for sodium ion batteries.
Keywords: capacity recovery; pulverization tolerance; semi‐coherent interfaces; sodium ion batteries; transmission electron microscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Rahman M. M., Sultana I., Chen Z., Srikanth M., Li L. H., Dai X. J., Chen Y., Nanoscale 2015, 7, 13088. - PubMed
-
- Xiao Y., Lee S. H., Sun Y.‐K., Adv. Energy Mater. 2017, 7, 1601329.
-
- Xiong X., Wang G., Lin Y., Wang Y., Ou X., Zheng F., Yang C., Wang J.‐H., Liu M., ACS Nano 2016, 10, 10953. - PubMed
-
- Sun J., Lee H.‐W., Pasta M., Yuan H., Zheng G., Sun Y., Li Y., Cui Y., Nat. Nanotechnol. 2015, 10, 980. - PubMed
-
- Liu Z., Lu T., Song T., Yu X.‐Y., Lou X. W., Paik U., Energy Environ. Sci. 2017, 10, 1576.
LinkOut - more resources
Full Text Sources
