Roles of Localized Electronic Structures Caused by π Degeneracy Due to Highly Symmetric Heavy Atom-Free Conjugated Molecular Crystals Leading to Efficient Persistent Room-Temperature Phosphorescence
- PMID: 31380211
- PMCID: PMC6661950
- DOI: 10.1002/advs.201900410
Roles of Localized Electronic Structures Caused by π Degeneracy Due to Highly Symmetric Heavy Atom-Free Conjugated Molecular Crystals Leading to Efficient Persistent Room-Temperature Phosphorescence
Abstract
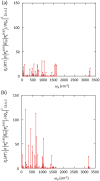
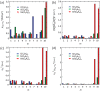
Conjugated molecular crystals with persistent room-temperature phosphorescence (RTP) are promising materials for sensing, security, and bioimaging applications. However, the electronic structures that lead to efficient persistent RTP are still unclear. Here, the electronic structures of tetraphenylmethane (C(C6H5)4), tetraphenylsilane (Si(C6H5)4), and tetraphenylgermane (Ge(C6H5)4) showing blue-green persistent RTP under ambient conditions are investigated. The persistent RTP of the crystals originates from minimization of triplet exciton quenching at room temperature not suppression of molecular vibrations. Localization of the highest occupied molecular orbitals (HOMOs) of the steric and highly symmetric conjugated crystal structures decreases the overlap of intermolecular HOMOs, minimizing triplet exciton migration, which accelerates defect quenching of triplet excitons. The localization of the HOMOs over the highly symmetric conjugated structures also induces moderate charge-transfer characteristics between high-order singlet excited states (S m ) and the ground state (S0). The combination of the moderate charge-transfer characteristics of the S m -S0 transition and local-excited state characteristics between the lowest excited triplet state and S0 accelerates the phosphorescence rate independent of the vibration-based nonradiative decay rate from the triplet state at room temperature. Thus, the decrease of triplet quenching and increase of phosphorescence rate caused by the HOMO localization contribute to the efficient persistent RTP of Ge(C6H5)4 crystals.
Keywords: aggregation induced emission; persistent room‐temperature phosphorescence; spin–orbit coupling; transfer integral; triplet exciton diffusion.
Conflict of interest statement
The author declares no conflict of interest.
Figures




References
-
- Baldo M. A., O'Brien D. F., You Y., Shoustikov A., Sibley S., Thompson M. E., Forrest S. R., Nature 1998, 395, 151.
-
- Baldo M. A., Lamansky S., Burrows P. E., Thompson M. E., Forrest S. R., Appl. Phys. Lett. 1999, 75, 4.
-
- Flamigni L., Barbieri A., Sabatini C., Ventura B., Barigelletti F., Top. Curr. Chem. 2007, 281, 143.
-
- Gareth Williams A. J., Top. Curr. Chem. 2007, 281, 205.
-
- Dolmans J. G. J. E. D., Fukumura D., Jain R. K., Nat. Rev. Cancer 2003, 3, 380. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
