New Insights Into the Golgi Stacking Proteins
- PMID: 31380369
- PMCID: PMC6660245
- DOI: 10.3389/fcell.2019.00131
New Insights Into the Golgi Stacking Proteins
Abstract
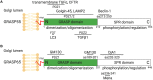
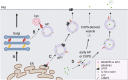
The Golgi stacking proteins, GRASP55 and GRASP65, are best known for their roles in Golgi structure formation. These peripheral Golgi proteins form trans-oligomers that hold the flat cisternal membranes into stacks. Depletion of both GRASP proteins in cells disrupts the Golgi stack structure, increases protein trafficking, but impairs accurate glycosylation, and sorting. Golgi unstacking by GRASPs depletion also reduces cell adhesion and migration in an integrin-dependent manner. In addition to Golgi structure formation and regulation of cellular activities, GRASPs, in particular GRASP55, have recently drawn attention in their roles in autophagy, and unconventional secretion. In autophagy, GRASP55 senses the energy level by O-GlcNAcylation, which regulates GRASP55 translocation from the Golgi to the autophagosome-lysosome interface, where it interacts with LC3 and LAMP2 to facilitate autophagosome-lysosome fusion. This newly discovered function of GRASP55 in autophagy may help explain its role in the stress-induced, autophagosome-dependent unconventional secretion. In this review, we summarize the emerging functions of the GRASP proteins, focusing on their roles in cell adhesion and migration, autophagy, unconventional secretion, as well as on novel GRASP-interacting proteins.
Keywords: GRASP55; GRASP65; Golgi; O-GlcNAcylation; autophagy; stacking; unconventional secretion.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

