Effects of Hexane Root Extract of Ferula hermonis Boiss. on Human Breast and Colon Cancer Cells: An In Vitro and In Vivo Study
- PMID: 31380416
- PMCID: PMC6662478
- DOI: 10.1155/2019/3079895
Effects of Hexane Root Extract of Ferula hermonis Boiss. on Human Breast and Colon Cancer Cells: An In Vitro and In Vivo Study
Abstract
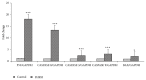
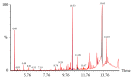
Breast and colon cancers are leading causes of cancer-related deaths globally. Plants are a potential source of natural products that may be used for the treatment of cancer. Ferula hermonis (FH) is reported to have diverse therapeutic effects. However, there are few reports on the in vitro anticancer potential of FH extract. Our results showed that the Ferula hermonis root hexane extract (FHRH) can induce dose-dependent cytotoxic effects in breast and colon cancer cells with MTT IC50 values of 18.2 and 25 μg/ml, respectively. The FHRH extract induced apoptosis in both breast and colon cancer cells; this was confirmed by light and nuclear staining, q-PCR, and caspase 3/7 activation. This study also demonstrated the antitumor activity of FHRH in 9,10-dimethylbenz[α]anthracene DMBA-induced rodent mammary tumor model. The GC/MS analysis revealed the presence of 3,5-Dimethylbenzenemethanol, Alpha-Bisabolol, Alpha-pinene, Beta-pinene, and Baccatin III that have various pharmacological potentials. Overall, the present study suggests that FHRH extract possesses anticancer potential which is mediated through apoptotic effects in MDA-MB-231 and LoVo cells. The present study also considered a basis for further investigations into the potential use of FHRH extract as an anticancer therapy for breast and colon cancers.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures






Similar articles
-
Phytochemistry and Pharmacology of Ferula hermonis Boiss. - A Review.Drug Res (Stuttg). 2017 Aug;67(8):437-446. doi: 10.1055/s-0043-109100. Epub 2017 May 18. Drug Res (Stuttg). 2017. PMID: 28521374 Review.
-
Ferula pseudalliacea induces apoptosis in human colorectal cancer HCT-116 cells via mitochondria-dependent pathway.Arch Physiol Biochem. 2019 Jul;125(3):284-291. doi: 10.1080/13813455.2018.1455710. Epub 2018 Mar 27. Arch Physiol Biochem. 2019. PMID: 29587544
-
Ferula hermonis: A Review of Current Use and Pharmacological Studies of its Sesquiterpene Ester Ferutinin.Curr Drug Targets. 2020;21(5):499-508. doi: 10.2174/1389450120666191029155053. Curr Drug Targets. 2020. PMID: 31663476 Review.
-
Assessment of Ferula hermonis Boiss fertility effects in immature female rats supported by quantification of ferutinin via HPLC and molecular docking.J Ethnopharmacol. 2022 May 10;289:115062. doi: 10.1016/j.jep.2022.115062. Epub 2022 Feb 1. J Ethnopharmacol. 2022. PMID: 35114339
-
Antibacterial activity and cytotoxicity of selected Egyptian medicinal plants.Planta Med. 2012 Jan;78(2):193-9. doi: 10.1055/s-0031-1280319. Epub 2011 Nov 4. Planta Med. 2012. PMID: 22057918
Cited by
-
Dietary Compounds for Targeting Prostate Cancer.Nutrients. 2019 Oct 8;11(10):2401. doi: 10.3390/nu11102401. Nutrients. 2019. PMID: 31597327 Free PMC article. Review.
-
The Inhibitory Effect and Mechanism of Ferula akitschkensis Volatile Oil on Gastric Cancer.Evid Based Complement Alternat Med. 2022 Mar 29;2022:5092742. doi: 10.1155/2022/5092742. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35392643 Free PMC article.
-
Cytotoxic Activity, Cell Cycle Inhibition, and Apoptosis-Inducing Potential of Athyrium hohenackerianum (Lady Fern) with Its Phytochemical Profiling.Evid Based Complement Alternat Med. 2022 Jun 2;2022:2055773. doi: 10.1155/2022/2055773. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35692581 Free PMC article.
-
Growth inhibitory effect of Leptospermum scoparium (manuka) chloroform extract on breast and liver cancer cell lines.J Adv Vet Anim Res. 2024 Jun 4;11(2):237-246. doi: 10.5455/javar.2024.k769. eCollection 2024 Jun. J Adv Vet Anim Res. 2024. PMID: 39101096 Free PMC article.
-
Ilimaquinone (Marine Sponge Metabolite) Induces Apoptosis in HCT-116 Human Colorectal Carcinoma Cells via Mitochondrial-Mediated Apoptosis Pathway.Mar Drugs. 2022 Sep 18;20(9):582. doi: 10.3390/md20090582. Mar Drugs. 2022. PMID: 36135771 Free PMC article.
References
-
- Fitzmaurice C., Allen C., Barber R. M., Barregard L., Bhutta Z. A., Brenner H., et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncology. 2017;3(4):524–548. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

