CNS-targeting pharmacological interventions for the metabolic syndrome
- PMID: 31380808
- PMCID: PMC6763237
- DOI: 10.1172/JCI129195
CNS-targeting pharmacological interventions for the metabolic syndrome
Abstract
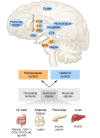
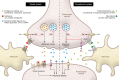
The metabolic syndrome (MetS) encompasses medical conditions such as obesity, hyperglycemia, high blood pressure, and dyslipidemia that are major drivers for the ever-increasing prevalence of type 2 diabetes, cardiovascular diseases, and certain types of cancer. At the core of clinical strategies against the MetS is weight loss, induced by bariatric surgery, lifestyle changes based on calorie reduction and exercise, or pharmacology. This Review summarizes the past, current, and future efforts of targeting the MetS by pharmacological agents. Major emphasis is given to drugs that target the CNS as a key denominator for obesity and its comorbid sequelae.
Conflict of interest statement
Figures

References
-
- Alberti KG, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

