Exploring Iron Withholding by the Innate Immune Protein Human Calprotectin
- PMID: 31381301
- PMCID: PMC6702060
- DOI: 10.1021/acs.accounts.9b00250
Exploring Iron Withholding by the Innate Immune Protein Human Calprotectin
Abstract
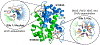
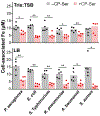
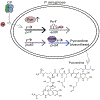
Calprotectin (CP) is a versatile player in the metal-withholding innate immune response, a process termed "nutritional immunity." CP is a heterooligomer of the polypeptides S100A8 and S100A9 and houses two transition-metal-binding sites at its S100A8/S100A9 heterodimer interface. During infection, CP is released from host cells and sequesters "bioavailable" transition metal ions in the extracellular space, thereby preventing microbial acquisition of these essential nutrients. For many years, the role of CP in nutritional immunity was interpreted in the contexts of Mn(II) and Zn(II) limitation, but recent work has broadened our understanding of its contributions to this process. We uncovered that CP provides a form of nutritional immunity that has previously received little attention: the battle between host and microbe for ferrous iron (Fe(II)). In this Account, we present our current understanding of Fe(II) coordination by CP and its role in Fe(II) withholding as well as considerations for future discovery. Nutritional immunity was first described in the context of host-microbe competition for ferric iron (Fe(III)). The battle for Fe(II) has received comparably little attention because the abundance of Fe(II) at infection sites and the importance of Fe(II) acquisition for microbial pathogenesis were recognized only recently. Several years ago, we discovered that human CP sequesters Fe(II) at its His6 site with subpicomolar affinity and thus hypothesized that it provides a means for Fe(II) limitation by the host during microbial infection. Fe(II) coordination by CP is unprecedented in biology because of its novel hexahistidine coordination sphere and its high-affinity binding, which surpasses that of other known Fe(II)-binding proteins. CP is also capable of shifting the Fe redox equilibrium by stabilizing Fe(II) in aerobic solution and can thereby sequester Fe in both reducing and nonreducing environments. These coordination chemistry studies allowed us to hypothesize that CP provides a means for Fe(II) limitation by the host during microbial infection. While investigating this putative Fe(II)-sequestering function, we discovered that CP withholds Fe from diverse bacterial pathogens. Recent studies by our lab and others of the bacterial pathogens Pseudomonas aeruginosa and Acinetobacter baumannii have shown that, by preventing sufficient Fe acquisition, CP induces Fe starvation responses in these organisms. As a result, CP affects bacterial virulence and metabolism. We also elucidated a complex interplay between CP and secondary metabolites produced by P. aeruginosa during the competition for Fe. Our work provides a foundation for understanding how CP affects Fe homeostasis during microbial infection. We believe that understanding how bacterial physiology is altered when challenged with Fe(II) withholding by CP will likely reveal crucial determinants of bacterial survival within the host.
Figures
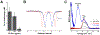
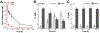
Similar articles
-
The human innate immune protein calprotectin induces iron starvation responses in Pseudomonas aeruginosa.J Biol Chem. 2019 Mar 8;294(10):3549-3562. doi: 10.1074/jbc.RA118.006819. Epub 2019 Jan 8. J Biol Chem. 2019. PMID: 30622135 Free PMC article.
-
The Human Innate Immune Protein Calprotectin Elicits a Multimetal Starvation Response in Pseudomonas aeruginosa.Microbiol Spectr. 2021 Oct 31;9(2):e0051921. doi: 10.1128/Spectrum.00519-21. Epub 2021 Sep 22. Microbiol Spectr. 2021. PMID: 34549997 Free PMC article.
-
Calprotectin-mediated survival of Staphylococcus aureus in coculture with Pseudomonas aeruginosa occurs without nutrient metal sequestration.mBio. 2025 May 14;16(5):e0384624. doi: 10.1128/mbio.03846-24. Epub 2025 Mar 28. mBio. 2025. PMID: 40152583 Free PMC article.
-
Bacterial Responses to Iron Withholding by Calprotectin.Biochemistry. 2021 Nov 16;60(45):3337-3346. doi: 10.1021/acs.biochem.1c00572. Epub 2021 Nov 5. Biochemistry. 2021. PMID: 34739212 Free PMC article. Review.
-
Transition Metal Sequestration by the Host-Defense Protein Calprotectin.Annu Rev Biochem. 2018 Jun 20;87:621-643. doi: 10.1146/annurev-biochem-062917-012312. Annu Rev Biochem. 2018. PMID: 29925260 Free PMC article. Review.
Cited by
-
Post-translational modifications on the metal-sequestering protein calprotectin.Biometals. 2023 Aug;36(4):817-828. doi: 10.1007/s10534-023-00493-x. Epub 2023 Feb 24. Biometals. 2023. PMID: 36826733 Free PMC article. Review.
-
Manganese Uptake, Mediated by SloABC and MntH, Is Essential for the Fitness of Streptococcus mutans.mSphere. 2020 Jan 8;5(1):e00764-19. doi: 10.1128/mSphere.00764-19. mSphere. 2020. PMID: 31915219 Free PMC article.
-
Steric Effects on the Chelation of Mn2+ and Zn2+ by Hexadentate Polyimidazole Ligands: Modeling Metal Binding by Calprotectin Site 2.Chemistry. 2023 Jul 3;29(37):e202300447. doi: 10.1002/chem.202300447. Epub 2023 May 11. Chemistry. 2023. PMID: 37067464 Free PMC article.
-
Negative Effect of Gst-35 on the Health Span of Caenorhabditis elegans Through Lysosomal Dysfunction via the Pmk-1 and Skr Genes.Aging Cell. 2025 Jun;24(6):e70016. doi: 10.1111/acel.70016. Epub 2025 Feb 13. Aging Cell. 2025. PMID: 39945496 Free PMC article.
-
Iron Acquisition by Bacterial Pathogens: Beyond Tris-Catecholate Complexes.Chembiochem. 2020 Jul 16;21(14):1955-1967. doi: 10.1002/cbic.201900778. Epub 2020 Apr 14. Chembiochem. 2020. PMID: 32180318 Free PMC article. Review.
References
-
- Weinberg ED, Nutritional immunity. Host’s attempt to withold iron from microbial invaders. J. Am. Med. Assoc. 1975, 231, 39–41. - PubMed
-
- Wilkinson MM; Busuttil A; Hayward C; Brock DJH; Dorin JA; Van Heyningen V, Expression pattern of two cystic fibrosis-associated calcium binding proteins in normal and abnormal tissues. J. Cell Sci 1988, 91, 221–230. - PubMed
-
- Bullock S; Hayward C; Manson J; Brock DJH; Raeburn JA, Quantitative immunoassays for diagnosis of carrier detection in cystic fibrosis. Clin. Genet 1982, 21, 336–341. - PubMed
-
- Dale I; Fagerhol MK; Naesgaard I, Purification and partial characterization of highly immunogenic human leucocyte protein, the L1 antigen. Eur. J. Biochem 1983, 134, 1–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

