Energy-efficient information transfer at thalamocortical synapses
- PMID: 31381555
- PMCID: PMC6695202
- DOI: 10.1371/journal.pcbi.1007226
Energy-efficient information transfer at thalamocortical synapses
Abstract
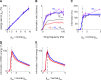
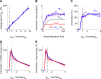
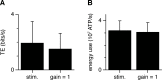
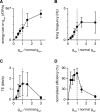
We have previously shown that the physiological size of postsynaptic currents maximises energy efficiency rather than information transfer across the retinothalamic relay synapse. Here, we investigate information transmission and postsynaptic energy use at the next synapse along the visual pathway: from relay neurons in the thalamus to spiny stellate cells in layer 4 of the primary visual cortex (L4SS). Using both multicompartment Hodgkin-Huxley-type simulations and electrophysiological recordings in rodent brain slices, we find that increasing or decreasing the postsynaptic conductance of the set of thalamocortical inputs to one L4SS cell decreases the energy efficiency of information transmission from a single thalamocortical input. This result is obtained in the presence of random background input to the L4SS cell from excitatory and inhibitory corticocortical connections, which were simulated (both excitatory and inhibitory) or injected experimentally using dynamic-clamp (excitatory only). Thus, energy efficiency is not a unique property of strong relay synapses: even at the relatively weak thalamocortical synapse, each of which contributes minimally to the output firing of the L4SS cell, evolutionarily-selected postsynaptic properties appear to maximise the information transmitted per energy used.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. JCBFM. 2001;21:1133–45. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

