Antifungal compounds from Streptomyces associated with attine ants also inhibit Leishmania donovani
- PMID: 31381572
- PMCID: PMC6695191
- DOI: 10.1371/journal.pntd.0007643
Antifungal compounds from Streptomyces associated with attine ants also inhibit Leishmania donovani
Abstract
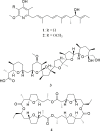
Bacterial strains isolated from attine ants showed activity against the insect specialized fungal pathogen Escovopsis and also against the human protozoan parasite Leishmania donovani. The bioassay guided fractionation of extracts from cultures of Streptomyces sp. ICBG292, isolated from the exoskeleton of Cyphomyrmex workers, led to the isolation of Mer-A2026B (1), piericidin-A1 (2) and nigericin (3). Nigericin (3) presented high activity against intracellular amastigotes of L. donovani (IC50 0.129 ± 0.008 μM). Streptomyces puniceus ICBG378, isolated from workers of Acromyrmex rugosus rugosus, produced dinactin (4) with potent anti-L. donovani activity against intracellular amastigotes (IC50 0.018 ± 0.003 μM). Compounds 3 and 4 showed good selectivity indexes, 88.91 and 656.11 respectively, and were more active than positive control, miltefosine. Compounds 1-4 were also active against some Escovopsis strains. Compounds 1 and 2 were also produced by Streptomyces sp. ICBG233, isolated from workers of Atta sexdens, and detected in ants' extracts by mass spectrometry, suggesting they are produced in the natural environment as defensive compounds involved in the symbiotic interaction.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
A single Streptomyces symbiont makes multiple antifungals to support the fungus farming ant Acromyrmex octospinosus.PLoS One. 2011;6(8):e22028. doi: 10.1371/journal.pone.0022028. Epub 2011 Aug 3. PLoS One. 2011. PMID: 21857911 Free PMC article.
-
Antileishmanial macrolides from ant-associated Streptomyces sp. ISID311.Bioorg Med Chem. 2021 Feb 15;32:116016. doi: 10.1016/j.bmc.2021.116016. Epub 2021 Jan 12. Bioorg Med Chem. 2021. PMID: 33493972 Free PMC article.
-
Candicidin-producing Streptomyces support leaf-cutting ants to protect their fungus garden against the pathogenic fungus Escovopsis.Proc Natl Acad Sci U S A. 2009 Mar 24;106(12):4742-6. doi: 10.1073/pnas.0812082106. Epub 2009 Mar 6. Proc Natl Acad Sci U S A. 2009. PMID: 19270078 Free PMC article.
-
Chemical warfare between fungus-growing ants and their pathogens.Curr Opin Chem Biol. 2020 Dec;59:172-181. doi: 10.1016/j.cbpa.2020.08.001. Epub 2020 Sep 17. Curr Opin Chem Biol. 2020. PMID: 32949983 Free PMC article. Review.
-
Symbiont recruitment versus ant-symbiont co-evolution in the attine ant-microbe symbiosis.Curr Opin Microbiol. 2012 Jun;15(3):269-77. doi: 10.1016/j.mib.2012.03.001. Epub 2012 Mar 23. Curr Opin Microbiol. 2012. PMID: 22445196 Review.
Cited by
-
Unravelling the potential of insects for medicinal purposes - A comprehensive review.Heliyon. 2023 Apr 29;9(5):e15938. doi: 10.1016/j.heliyon.2023.e15938. eCollection 2023 May. Heliyon. 2023. PMID: 37206028 Free PMC article. Review.
-
HAS 1: A natural product from soil-isolated Streptomyces species with potent activity against cutaneous leishmaniasis caused by Leishmania tropica.Front Pharmacol. 2022 Oct 10;13:1023114. doi: 10.3389/fphar.2022.1023114. eCollection 2022. Front Pharmacol. 2022. PMID: 36299890 Free PMC article.
-
Isolation of Actinobacteria from Date Palm Rhizosphere with Enzymatic, Antimicrobial, Antioxidant, and Protein Denaturation Inhibitory Activities.Biomolecules. 2025 Jan 5;15(1):65. doi: 10.3390/biom15010065. Biomolecules. 2025. PMID: 39858459 Free PMC article.
-
Microorganisms as a Potential Source of Molecules to Control Trypanosomatid Diseases.Molecules. 2021 Mar 4;26(5):1388. doi: 10.3390/molecules26051388. Molecules. 2021. PMID: 33806654 Free PMC article. Review.
-
Lenzimycins A and B, Metabolites With Antibacterial Properties From Brevibacillus sp. Associated With the Dung Beetle Onthophagus lenzii.Front Microbiol. 2020 Oct 30;11:599911. doi: 10.3389/fmicb.2020.599911. eCollection 2020. Front Microbiol. 2020. PMID: 33193283 Free PMC article.
References
-
- World Health Organization. Leishmaniasis. 2019. https://www.who.int/leishmaniasis/en/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

