Combination antioxidant therapy prevents epileptogenesis and modifies chronic epilepsy
- PMID: 31382215
- PMCID: PMC6692059
- DOI: 10.1016/j.redox.2019.101278
Combination antioxidant therapy prevents epileptogenesis and modifies chronic epilepsy
Abstract
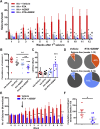
Many epilepsies are acquired conditions following an insult to the brain such as a prolonged seizure, traumatic brain injury or stroke. The generation of reactive oxygen species (ROS) and induction of oxidative stress are common sequelae of such brain insults and have been shown to contribute to neuronal death and the development of epilepsy. Here, we show that combination therapy targeting the generation of ROS through NADPH oxidase inhibition and the endogenous antioxidant system through nuclear factor erythroid 2-related factor 2 (Nrf2) activation prevents excessive ROS accumulation, mitochondrial depolarisation and neuronal death during in vitro seizure-like activity. Moreover, this combination therapy prevented the development of spontaneous seizures in 40% of animals following status epilepticus (70% of animals were seizure free after 8 weeks) and modified the severity of epilepsy when given to chronic epileptic animals.
Keywords: Epileptogenesis; Keap1-Nrf2 pathway; NADPH oxidase; Oxidative stress; Spontaneous seizures.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

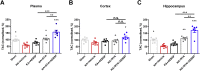
Similar articles
-
KEAP1 inhibition is neuroprotective and suppresses the development of epilepsy.Brain. 2018 May 1;141(5):1390-1403. doi: 10.1093/brain/awy071. Brain. 2018. PMID: 29538645
-
Status epilepticus results in persistent overproduction of reactive oxygen species, inhibition of which is neuroprotective.Neuroscience. 2015 Sep 10;303:160-5. doi: 10.1016/j.neuroscience.2015.07.005. Epub 2015 Jul 7. Neuroscience. 2015. PMID: 26162241
-
Post-treatment of an NADPH oxidase inhibitor prevents seizure-induced neuronal death.Brain Res. 2013 Mar 7;1499:163-72. doi: 10.1016/j.brainres.2013.01.007. Epub 2013 Jan 10. Brain Res. 2013. PMID: 23313582
-
Antioxidants Targeting Mitochondrial Oxidative Stress: Promising Neuroprotectants for Epilepsy.Oxid Med Cell Longev. 2020 Nov 25;2020:6687185. doi: 10.1155/2020/6687185. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 33299529 Free PMC article. Review.
-
Reactive oxygen species in status epilepticus.Epilepsy Behav. 2019 Dec;101(Pt B):106410. doi: 10.1016/j.yebeh.2019.07.011. Epub 2019 Aug 1. Epilepsy Behav. 2019. PMID: 31378559 Review.
Cited by
-
Unifying mechanism behind the onset of acquired epilepsy.Trends Pharmacol Sci. 2022 Feb;43(2):87-96. doi: 10.1016/j.tips.2021.11.009. Epub 2021 Dec 6. Trends Pharmacol Sci. 2022. PMID: 34887128 Free PMC article. Review.
-
Accumulation of advanced oxidation protein products aggravates bone-fat imbalance during skeletal aging.J Orthop Translat. 2025 Jan 21;51:24-36. doi: 10.1016/j.jot.2024.12.010. eCollection 2025 Mar. J Orthop Translat. 2025. PMID: 39902100 Free PMC article.
-
Unlocking the Potential of Freeze-Dried Broccoli Powder: A Novel Approach to Enhancing Cognitive Resilience in Temporal Lobe Epilepsy.Food Sci Nutr. 2025 Mar 8;13(3):e70079. doi: 10.1002/fsn3.70079. eCollection 2025 Mar. Food Sci Nutr. 2025. PMID: 40061295 Free PMC article.
-
Repurposed molecules for antiepileptogenesis: Missing an opportunity to prevent epilepsy?Epilepsia. 2020 Mar;61(3):359-386. doi: 10.1111/epi.16450. Epilepsia. 2020. PMID: 32196665 Free PMC article. Review.
-
Seizure-mediated iron accumulation and dysregulated iron metabolism after status epilepticus and in temporal lobe epilepsy.Acta Neuropathol. 2021 Oct;142(4):729-759. doi: 10.1007/s00401-021-02348-6. Epub 2021 Jul 22. Acta Neuropathol. 2021. PMID: 34292399 Free PMC article.
References
-
- Zhang R. Nrf2-a promising therapeutic target for defensing against oxidative stress in stroke. Mol. Neurobiol. 2017;54(8):6006–6017. - PubMed
-
- Bhatti J. Systematic review of human and animal studies examining the efficacy and safety of N-acetylcysteine (NAC) and N-acetylcysteine amide (NACA) in traumatic brain injury: impact on neurofunctional outcome and biomarkers of oxidative stress and inflammation. Front. Neurol. 2017;8:744. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

