Immunogenicity and Efficacy Evaluation of Subunit Astrovirus Vaccines
- PMID: 31382451
- PMCID: PMC6789684
- DOI: 10.3390/vaccines7030079
Immunogenicity and Efficacy Evaluation of Subunit Astrovirus Vaccines
Abstract
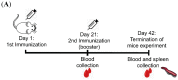
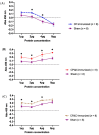
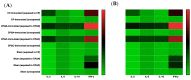
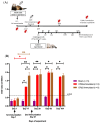
A full understanding of the immune response to astrovirus (AstV) infection is required to treat and control AstV-induced gastroenteritis. Relative contributions of each arm of the immune system in restricting AstV infection remain unknown. In this study, two novel subunit AstV vaccines derived from capsid protein (CP) of mink AstV (MAstV) such as CPΔN (spanning amino acids 161-775) and CPΔC (spanning amino acids 1-621) were evaluated. Their immunogenicity and cytokine production in mice, as well as protective efficacy in mink litters via maternal immunization, were studied. Truncated CPs induced higher levels of serum anti-CP antibodies than CP, with the highest level for CPΔN. No seronegativity was detected after booster immunization with either AstV CP truncates in both mice and mink. All mink moms stayed seropositive during the entire 104-day study. Furthermore, lymphoproliferation responses and Th1/Th2 cytokine induction of mice splenocytes ex vivo re-stimulated by truncated CPs were significantly higher than those by CP, with the highest level for CPΔN. Immunization of mink moms with truncated CPs could suppress virus shedding and clinical signs in their litters during a 51-day study after challenge with a heterogeneous MAstV strain. Collectively, AstV truncated CPs exhibit better parameters for protection than full-length CP.
Keywords: astrovirus; capsid protein; immunogenicity; infection; subunit vaccine.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures


Similar articles
-
The first evidence of shaking mink syndrome-astrovirus associated encephalitis in farmed minks, China.Transbound Emerg Dis. 2022 Nov;69(6):3979-3984. doi: 10.1111/tbed.14693. Epub 2022 Oct 6. Transbound Emerg Dis. 2022. PMID: 36057957
-
Construction and Immunogenicity Analysis of Whole-Gene Mutation DNA Vaccine of Aleutian Mink Virus Isolated Virulent Strain.Viral Immunol. 2018 Jan/Feb;31(1):69-77. doi: 10.1089/vim.2017.0044. Epub 2017 Aug 22. Viral Immunol. 2018. PMID: 28829241
-
Development and evaluation of two subunit vaccine candidates containing antigens of hepatitis E virus, rotavirus, and astrovirus.Sci Rep. 2016 May 19;6:25735. doi: 10.1038/srep25735. Sci Rep. 2016. PMID: 27194006 Free PMC article.
-
The Immune Response to Astrovirus Infection.Viruses. 2016 Dec 30;9(1):1. doi: 10.3390/v9010001. Viruses. 2016. PMID: 28042824 Free PMC article. Review.
-
Use of interleukin 12 to enhance the cellular immune response of swine to an inactivated herpesvirus vaccine.Adv Vet Med. 1999;41:447-61. doi: 10.1016/s0065-3519(99)80034-2. Adv Vet Med. 1999. PMID: 9890035 Review.
Cited by
-
Nanocarrier vaccines for SARS-CoV-2.Adv Drug Deliv Rev. 2021 Apr;171:215-239. doi: 10.1016/j.addr.2021.01.002. Epub 2021 Jan 9. Adv Drug Deliv Rev. 2021. PMID: 33428995 Free PMC article.
-
Vaccines against gastroenteritis, current progress and challenges.Gut Microbes. 2020 Nov 1;11(6):1486-1517. doi: 10.1080/19490976.2020.1770666. Epub 2020 Jun 18. Gut Microbes. 2020. PMID: 32552414 Free PMC article. Review.
-
Molecular Characterization and Determination of Relative Cytokine Expression in Naturally Infected Day-Old Chicks with Chicken Astrovirus Associated to White Chick Syndrome.Animals (Basel). 2020 Jul 14;10(7):1195. doi: 10.3390/ani10071195. Animals (Basel). 2020. PMID: 32674433 Free PMC article.
-
Mechanistic insights from the review and evaluation of ayurvedic herbal medicines for the prevention and management of COVID-19 patients.J Herb Med. 2022 Mar;32:100554. doi: 10.1016/j.hermed.2022.100554. Epub 2022 Feb 18. J Herb Med. 2022. PMID: 35251909 Free PMC article.
-
Molecular study of human astrovirus in Egyptian children with acute gastroenteritis.Germs. 2020 Sep 1;10(4):167-173. doi: 10.18683/germs.2020.1202. eCollection 2020 Sep. Germs. 2020. PMID: 33134194 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

