Formation Mechanism of Ion Channel in Channelrhodopsin-2: Molecular Dynamics Simulation and Steering Molecular Dynamics Simulations
- PMID: 31382458
- PMCID: PMC6695816
- DOI: 10.3390/ijms20153780
Formation Mechanism of Ion Channel in Channelrhodopsin-2: Molecular Dynamics Simulation and Steering Molecular Dynamics Simulations
Abstract
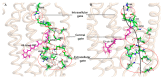
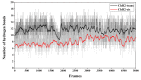
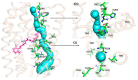
Channelrhodopsin-2 (ChR2) is a light-activated and non-selective cationic channel protein that can be easily expressed in specific neurons to control neuronal activity by light. Although ChR2 has been extensively used as an optogenetic tool in neuroscience research, the molecular mechanism of cation channel formation following retinal photoisomerization in ChR2 is not well understood. In this paper, studies of the closed and opened state ChR2 structures are presented. The formation of the cationic channel is elucidated in atomic detail using molecular dynamics simulations on the all-trans-retinal (ChR2-trans) configuration of ChR2 and its isomerization products, 13-cis-retinal (ChR2-cis) configuration, respectively. Photoisomerization of the retinal-chromophore causes the destruction of interactions among the crucial residues (e.g., E90, E82, N258, and R268) around the channel and the extended H-bond network mediated by numerous water molecules, which opens the pore. Steering molecular dynamics (SMD) simulations show that the electrostatic interactions at the binding sites in intracellular gate (ICG) and central gate (CG) can influence the transmembrane transport of Na+ in ChR2-cis obviously. Potential of mean force (PMF) constructed by SMD and umbrella sampling also found the existing energy wells at these two binding sites during the transportation of Na+. These wells partly hinder the penetration of Na+ into cytoplasm through the ion channel. This investigation provides a theoretical insight on the formation mechanism of ion channels and the mechanism of ion permeation.
Keywords: Steering Molecular Dynamics Simulations; channelrhodopsin-2; hydrogen bond network; ion channel; photoisomerization.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures
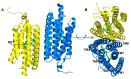






Similar articles
-
Retinal isomerization and water-pore formation in channelrhodopsin-2.Proc Natl Acad Sci U S A. 2018 Apr 3;115(14):3557-3562. doi: 10.1073/pnas.1700091115. Epub 2018 Mar 19. Proc Natl Acad Sci U S A. 2018. PMID: 29555736 Free PMC article.
-
Unifying photocycle model for light adaptation and temporal evolution of cation conductance in channelrhodopsin-2.Proc Natl Acad Sci U S A. 2019 May 7;116(19):9380-9389. doi: 10.1073/pnas.1818707116. Epub 2019 Apr 19. Proc Natl Acad Sci U S A. 2019. PMID: 31004059 Free PMC article.
-
Nonadiabatic Photodynamics of Retinal Protonated Schiff Base in Channelrhodopsin 2.J Phys Chem Lett. 2019 Jun 6;10(11):2862-2868. doi: 10.1021/acs.jpclett.9b00701. Epub 2019 May 16. J Phys Chem Lett. 2019. PMID: 31083920
-
Channelrhodopsins: a bioinformatics perspective.Biochim Biophys Acta. 2014 May;1837(5):643-55. doi: 10.1016/j.bbabio.2013.11.005. Epub 2013 Nov 16. Biochim Biophys Acta. 2014. PMID: 24252597 Review.
-
Channelrhodopsin unchained: structure and mechanism of a light-gated cation channel.Biochim Biophys Acta. 2014 May;1837(5):626-42. doi: 10.1016/j.bbabio.2013.10.014. Epub 2013 Nov 7. Biochim Biophys Acta. 2014. PMID: 24212055 Review.
Cited by
-
Optogenetics: Illuminating the Future of Hearing Restoration and Understanding Auditory Perception.Curr Gene Ther. 2024;24(3):208-216. doi: 10.2174/0115665232269742231213110937. Curr Gene Ther. 2024. PMID: 38676313 Review.
-
Pore-Opening and Ion-Conduction Mechanism in Channelrhodopsins C1C2, ChR2, and iChloC by Computational Electrophysiology and Constant-pH Simulations.J Chem Inf Model. 2025 Jun 9;65(11):5649-5661. doi: 10.1021/acs.jcim.5c00356. Epub 2025 May 29. J Chem Inf Model. 2025. PMID: 40439938 Free PMC article.
-
Molecular Dynamics Simulation of Transmembrane Transport of Chloride Ions in Mutants of Channelrhodopsin.Biomolecules. 2019 Dec 10;9(12):852. doi: 10.3390/biom9120852. Biomolecules. 2019. PMID: 31835536 Free PMC article.
-
Time-resolved photoacoustics of channelrhodopsins: early energetics and light-driven volume changes.Photochem Photobiol Sci. 2023 Mar;22(3):477-486. doi: 10.1007/s43630-022-00327-8. Epub 2022 Oct 23. Photochem Photobiol Sci. 2023. PMID: 36273368
-
The short-term plasticity of VIP interneurons in motor cortex.Front Synaptic Neurosci. 2024 Aug 29;16:1433977. doi: 10.3389/fnsyn.2024.1433977. eCollection 2024. Front Synaptic Neurosci. 2024. PMID: 39267890 Free PMC article.
References
-
- Georg N., Martin B., Liewald J.F., Nona A., Ernst B., Alexander G. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr. Biol. 2005;15:2279–2284. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

