Multiple Modulation of Acid-Sensing Ion Channel 1a by the Alkaloid Daurisoline
- PMID: 31382492
- PMCID: PMC6722837
- DOI: 10.3390/biom9080336
Multiple Modulation of Acid-Sensing Ion Channel 1a by the Alkaloid Daurisoline
Abstract
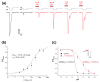
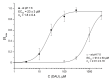
Acid-sensing ion channels (ASICs) are proton-gated sodium-selective channels that are expressed in the peripheral and central nervous systems. ASIC1a is one of the most intensively studied isoforms due to its importance and wide representation in organisms, but it is still largely unexplored as a target for therapy. In this study, we demonstrated response of the ASIC1a to acidification in the presence of the daurisoline (DAU) ligand. DAU alone did not activate the channel, but in combination with protons, it produced the second peak component of the ASIC1a current. This second peak differs from the sustained component (which is induced by RF-amide peptides), as the second (DAU-induced) peak is completely desensitized, with the same kinetics as the main peak. The co-application of DAU and mambalgin-2 indicated that their binding sites do not overlap. Additionally, we found an asymmetry in the pH activation curve of the channel, which was well-described by a mathematical model based on the multiplied probabilities of protons binding with a pool of high-cooperative sites and a single proton binding with a non-cooperative site. In this model, DAU targeted the pool of high-cooperative sites and, when applied with protons, acted as an inhibitor of ASIC1a activation. Moreover, DAU's occupation of the same binding site most probably reverses the channel from steady-state desensitization in the pH 6.9-7.3 range. DAU features disclose new opportunities in studies of ASIC structure and function.
Keywords: ASIC1a channels; channel desensitization; daurisoline; potentiator.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

