Real-Time 3D Single Particle Tracking: Towards Active Feedback Single Molecule Spectroscopy in Live Cells
- PMID: 31382495
- PMCID: PMC6695621
- DOI: 10.3390/molecules24152826
Real-Time 3D Single Particle Tracking: Towards Active Feedback Single Molecule Spectroscopy in Live Cells
Abstract
Single molecule fluorescence spectroscopy has been largely implemented using methods which require tethering of molecules to a substrate in order to make high temporal resolution measurements. However, the act of tethering a molecule requires that the molecule be removed from its environment. This is especially perturbative when measuring biomolecules such as enzymes, which may rely on the non-equilibrium and crowded cellular environment for normal function. A method which may be able to un-tether single molecule fluorescence spectroscopy is real-time 3D single particle tracking (RT-3D-SPT). RT-3D-SPT uses active feedback to effectively lock-on to freely diffusing particles so they can be measured continuously with up to photon-limited temporal resolution over large axial ranges. This review gives an overview of the various active feedback 3D single particle tracking methods, highlighting specialized detection and excitation schemes which enable high-speed real-time tracking. Furthermore, the combination of these active feedback methods with simultaneous live-cell imaging is discussed. Finally, the successes in real-time 3D single molecule tracking (RT-3D-SMT) thus far and the roadmap going forward for this promising family of techniques are discussed.
Keywords: active feedback tracking; real-time 3D single particle tracking; single molecule spectroscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



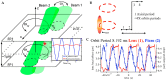
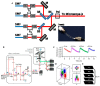

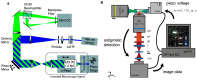
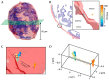

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

