Removal of Chromium from a Contaminated Soil Using Oxalic Acid, Citric Acid, and Hydrochloric Acid: Dynamics, Mechanisms, and Concomitant Removal of Non-Targeted Metals
- PMID: 31382525
- PMCID: PMC6696345
- DOI: 10.3390/ijerph16152771
Removal of Chromium from a Contaminated Soil Using Oxalic Acid, Citric Acid, and Hydrochloric Acid: Dynamics, Mechanisms, and Concomitant Removal of Non-Targeted Metals
Abstract
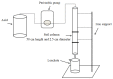
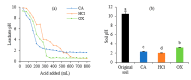
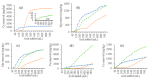
Soil leaching is an effective remediation technique using agents to leach the target pollutants from the soil. However, the dynamics and mechanisms for leaching of Cr and other non-pollutant metals from Cr-contaminated soils are not yet well understood. Here, column leaching experiments were conducted to determine the effect of hydrochloric acid (HCl), citric acid (CA), and oxalic acid (OX) on the leaching of Cr, as well as of Ca, Mg, Fe, and Mn, from a soil contaminated by a Cr slag heap. Acid leaching decreased soil pH and enhanced the mobility of all the surveyed metals. Leaching dynamics varied with both metals and acids. OX had the highest removal rates for Cr, Fe, Mn, and Mg, but had the poorest ability to leach Ca. HCl leached the largest amount of Ca, while CA leached similar amounts of Mg and Mn to OX, and similar amounts of Fe and Cr to HCl. Cr in the leachates was correlated with Ca, Mg, Fe, and Mn. Cr mainly interacted with soil mineral components and showed a punctate distribution in soil particles. The X-ray diffraction (XRD), scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM-EDS), and X-ray photoelectron spectroscopy (XPS) analyses showed soil mineralogical and morphological properties were differently altered after leaching by different acids. Complexation of Cr(III), competitive desorption, and reduction of Cr(VI) make significant contribution to Cr leaching by organic acids. In conclusion, OX can be applied in leaching remediation of Cr-contaminated soil, but the concomitant removal of other non-targeted metals should be taken into account because of the loss of soil minerals and fertility.
Keywords: chemical leaching; chromium; column experiment; soil remediation; soil washing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Yao Z., Li J., Xie H., Yu C. Review on remediation technologies of soil contaminated by heavy metals. Procedia Environ. Sci. 2012;16:722–729. doi: 10.1016/j.proenv.2012.10.099. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

