Cartilage and Bone Destruction in Arthritis: Pathogenesis and Treatment Strategy: A Literature Review
- PMID: 31382539
- PMCID: PMC6721572
- DOI: 10.3390/cells8080818
Cartilage and Bone Destruction in Arthritis: Pathogenesis and Treatment Strategy: A Literature Review
Abstract
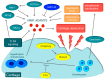
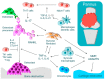
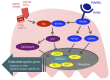
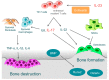
Arthritis is inflammation of the joints accompanied by osteochondral destruction. It can take many forms, including osteoarthritis, rheumatoid arthritis, and psoriatic arthritis. These diseases share one commonality-osteochondral destruction based on inflammation. The background includes a close interaction between osseous tissues and immune cells through various inflammatory cytokines. However, the tissues and cytokines that play major roles are different in each disease, and as a result, the mechanism of osteochondral destruction also differs. In recent years, there have been many findings regarding not only extracellular signaling pathways but also intracellular signaling pathways. In particular, we anticipate that the intracellular signals of osteoclasts, which play a central role in bone destruction, will become novel therapeutic targets. In this review, we have summarized the pathology of arthritis and the latest findings on the mechanism of osteochondral destruction, as well as present and future therapeutic strategies for these targets.
Keywords: arthritis; cartilage and bone destruction; osteoarthritis; osteoclast; psoriatic arthritis; rheumatoid arthritis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Vos T., Flaxman A.D., Naghavi M., Lozano R., Michaud C., Ezzati M., Shibuya K., Salomon J.A., Abdalla S., Aboyans V., et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

