The Role of the Brain in the Pathogenesis and Physiology of Polycystic Ovary Syndrome (PCOS)
- PMID: 31382541
- PMCID: PMC6722593
- DOI: 10.3390/medsci7080084
The Role of the Brain in the Pathogenesis and Physiology of Polycystic Ovary Syndrome (PCOS)
Abstract
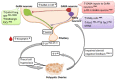
Polycystic ovary syndrome (PCOS) is a common reproductive endocrine disorder, affecting at least 10% of women of reproductive age. PCOS is typically characterized by the presence of at least two of the three cardinal features of hyperandrogenemia (high circulating androgen levels), oligo- or anovulation, and cystic ovaries. Hyperandrogenemia increases the severity of the condition and is driven by increased luteinizing hormone (LH) pulse secretion from the pituitary. Indeed, PCOS women display both elevated mean LH levels, as well as an elevated frequency of LH pulsatile secretion. The abnormally high LH pulse frequency, reflective of a hyperactive gonadotropin-releasing hormone (GnRH) neural circuit, suggests a neuroendocrine basis to either the etiology or phenotype of PCOS. Several studies in preclinical animal models of PCOS have demonstrated alterations in GnRH neurons and their upstream afferent neuronal circuits. Some rodent PCOS models have demonstrated an increase in GnRH neuron activity that correlates with an increase in stimulatory GABAergic innervation and postsynaptic currents onto GnRH neurons. Additional studies have identified robust increases in hypothalamic levels of kisspeptin, another potent stimulator of GnRH neurons. This review outlines the different brain and neuroendocrine changes in the reproductive axis observed in PCOS animal models, discusses how they might contribute to either the etiology or adult phenotype of PCOS, and considers parallel findings in PCOS women.
Keywords: GABA; GnRH; Kiss1; Kisspeptin; LH; PCOS; androgen; brain; neuroendocrine; pulses.
Conflict of interest statement
The authors report no conflict of interest.
Figures