Histamine Is an Inducer of the Heat Shock Response in SOD1-G93A Models of ALS
- PMID: 31382568
- PMCID: PMC6696457
- DOI: 10.3390/ijms20153793
Histamine Is an Inducer of the Heat Shock Response in SOD1-G93A Models of ALS
Abstract
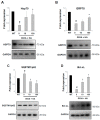
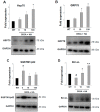
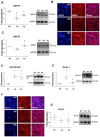
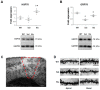
(1) Background: Amyotrophic lateral sclerosis (ALS) is a multifactorial non-cell autonomous disease where activation of microglia and astrocytes largely contributes to motor neurons death. Heat shock proteins have been demonstrated to promote neuronal survival and exert a strong anti-inflammatory action in glia. Having previously shown that the pharmacological increase of the histamine content in the central nervous system (CNS) of SOD1-G93A mice decreases neuroinflammation, reduces motor neuron death, and increases mice life span, here we examined whether this effect could be mediated by an enhancement of the heat shock response. (2) Methods: Heat shock protein expression was analyzed in vitro and in vivo. Histamine was provided to primary microglia and NSC-34 motor neurons expressing the SOD1-G93A mutation. The brain permeable histamine precursor histidine was chronically administered to symptomatic SOD1-G93A mice. Spine density was measured by Golgi-staining in motor cortex of histidine-treated SOD1-G93A mice. (3) Results: We demonstrate that histamine activates the heat shock response in cultured SOD1-G93A microglia and motor neurons. In SOD1-G93A mice, histidine augments the protein content of GRP78 and Hsp70 in spinal cord and cortex, where the treatment also rescues type I motor neuron dendritic spine loss. (4) Conclusion: Besides the established histaminergic neuroprotective and anti-inflammatory effects, the induction of the heat shock response in the SOD1-G93A model by histamine confirms the importance of this pathway in the search for successful therapeutic solutions to treat ALS.
Keywords: SOD1-G93A; dendritic spines; heat shock proteins; histamine; microglia; motor neurons.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

