Optical Investigation of Action Potential and Calcium Handling Maturation of hiPSC-Cardiomyocytes on Biomimetic Substrates
- PMID: 31382622
- PMCID: PMC6695920
- DOI: 10.3390/ijms20153799
Optical Investigation of Action Potential and Calcium Handling Maturation of hiPSC-Cardiomyocytes on Biomimetic Substrates
Abstract
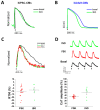
Cardiomyocytes from human induced pluripotent stem cells (hiPSC-CMs) are the most promising human source with preserved genetic background of healthy individuals or patients. This study aimed to establish a systematic procedure for exploring development of hiPSC-CM functional output to predict genetic cardiomyopathy outcomes and identify molecular targets for therapy. Biomimetic substrates with microtopography and physiological stiffness can overcome the immaturity of hiPSC-CM function. We have developed a custom-made apparatus for simultaneous optical measurements of hiPSC-CM action potential and calcium transients to correlate these parameters at specific time points (day 60, 75 and 90 post differentiation) and under inotropic interventions. In later-stages, single hiPSC-CMs revealed prolonged action potential duration, increased calcium transient amplitude and shorter duration that closely resembled those of human adult cardiomyocytes from fresh ventricular tissue of patients. Thus, the major contribution of sarcoplasmic reticulum and positive inotropic response to β-adrenergic stimulation are time-dependent events underlying excitation contraction coupling (ECC) maturation of hiPSC-CM; biomimetic substrates can promote calcium-handling regulation towards adult-like kinetics. Simultaneous optical recordings of long-term cultured hiPSC-CMs on biomimetic substrates favor high-throughput electrophysiological analysis aimed at testing (mechanistic hypothesis on) disease progression and pharmacological interventions in patient-derived hiPSC-CMs.
Keywords: action potential; calcium handling; cardiomyocytes; fluorescence; human induced pluripotent stem cells; hydrogels; long-term culture; maturation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- van den Berg C.W., Okawa S., Chuva de Sousa Lopes S.M., van Iperen L., Passier R., Braam S.R., Tertoolen L.G., del Sol A., Davis R.P., Mummery C.L. Transcriptome of human foetal heart compared with cardiomyocytes from pluripotent stem cells. Development. 2015;142:3231–3238. doi: 10.1242/dev.123810. - DOI - PubMed

