Appressorium: The Breakthrough in Dikarya
- PMID: 31382649
- PMCID: PMC6787622
- DOI: 10.3390/jof5030072
Appressorium: The Breakthrough in Dikarya
Abstract
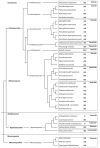
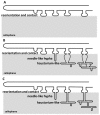
Phytopathogenic and mycorrhizal fungi often penetrate living hosts by using appressoria and related structures. The differentiation of similar structures in saprotrophic fungi to penetrate dead plant biomass has seldom been investigated and has been reported only in the model fungus Podospora anserina. Here, we report on the ability of many saprotrophs from a large range of taxa to produce appressoria on cellophane. Most Ascomycota and Basidiomycota were able to form appressoria. In contrast, none of the three investigated Mucoromycotina was able to differentiate such structures. The ability of filamentous fungi to differentiate appressoria no longer belongs solely to pathogenic or mutualistic fungi, and this raises the question of the evolutionary origin of the appressorium in Eumycetes.
Keywords: Eumycetes; appressorium; biomass degradation; cellophane; infection cushion; penetration; saprotrophic fungi.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Xie N., Chapeland-Leclerc F., Silar P., Ruprich-Robert G. Systematic gene deletions evidences that laccases are involved in several stages of wood degradation in the filamentous fungus Podospora anserina: Wood degradation by laccases in Podospora anserina. Environ. Microbiol. 2014;16:141–161. doi: 10.1111/1462-2920.12253. - DOI - PubMed
LinkOut - more resources
Full Text Sources

