Alcohol Participates in the Synthesis of Functionalized Coumarin-Fused Pyrazolo[3,4- b]Pyridine from a One-Pot Three-Component Reaction
- PMID: 31382711
- PMCID: PMC6696490
- DOI: 10.3390/molecules24152835
Alcohol Participates in the Synthesis of Functionalized Coumarin-Fused Pyrazolo[3,4- b]Pyridine from a One-Pot Three-Component Reaction
Abstract
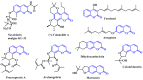
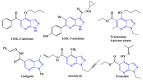
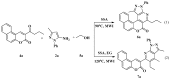
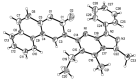
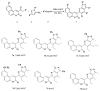
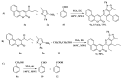
A concise and efficient approach to synthesizing coumarin-fused pyrazolo[3,4-b]pyridine via silica sulfuric acid (SSA) catalyzed three-component domino reaction under microwave irradiation has been demonstrated. Participation of various alcohols in construction of coumarin derivatives has been described for the first time. Short reaction time, high yields, one-pot procedure, usage of eco-friendly catalyst, and solvent are the key features of this method.
Keywords: coumarin; pyrazolo[3,4-b]pyridine; silica sulfuric acid; synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
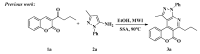

Similar articles
-
Multicomponent approaches to 8-carboxylnaphthyl-functionalized pyrazolo[3,4-b]pyridine derivatives.Org Biomol Chem. 2012 Jan 28;10(4):724-8. doi: 10.1039/c1ob06624b. Epub 2011 Dec 14. Org Biomol Chem. 2012. PMID: 22167248
-
An efficient one-pot synthesis of functionalized chromeno[4,3-b]pyridine derivatives under catalyst-free conditions.Mol Divers. 2017 May;21(2):293-304. doi: 10.1007/s11030-016-9723-6. Epub 2017 Jan 31. Mol Divers. 2017. PMID: 28144767
-
Synthesis of pyrazolo[3,4-b]pyridines under microwave irradiation in multi-component reactions and their antitumor and antimicrobial activities - Part 1.Eur J Med Chem. 2012 Feb;48:92-6. doi: 10.1016/j.ejmech.2011.11.038. Epub 2011 Dec 1. Eur J Med Chem. 2012. PMID: 22178093
-
Recent developments in utility of green multi-component reactions for the efficient synthesis of polysubstituted pyrans, thiopyrans, pyridines, and pyrazoles.Mol Divers. 2015 Aug;19(3):625-51. doi: 10.1007/s11030-015-9594-2. Epub 2015 Apr 17. Mol Divers. 2015. PMID: 25894364 Review.
-
Greener Syntheses of Coumarin Derivatives Using Magnetic Nanocatalysts: Recent Advances.Top Curr Chem (Cham). 2022 Nov 12;381(1):1. doi: 10.1007/s41061-022-00407-4. Top Curr Chem (Cham). 2022. PMID: 36370211 Review.
Cited by
-
Recent advances in pyrazolo[3,4-b]pyridine chemistry: synthesis techniques and biological activity.Mol Divers. 2025 Jun 25. doi: 10.1007/s11030-025-11231-5. Online ahead of print. Mol Divers. 2025. PMID: 40560489 Review.
-
Modern Strategies for Heterocycle Synthesis.Molecules. 2020 May 27;25(11):2476. doi: 10.3390/molecules25112476. Molecules. 2020. PMID: 32471057 Free PMC article.
-
Streptomyces pactum-Mediated Bioprocess for Silver Nanoparticles: A Green Catalyst for One-Pot Synthesis of Thiazolidine 4‑Ones and Fluorinated Derivatives.ACS Omega. 2025 May 20;10(21):20993-21007. doi: 10.1021/acsomega.4c03444. eCollection 2025 Jun 3. ACS Omega. 2025. PMID: 40488053 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

