Class effects of SGLT2 inhibitors on cardiorenal outcomes
- PMID: 31382965
- PMCID: PMC6683461
- DOI: 10.1186/s12933-019-0903-4
Class effects of SGLT2 inhibitors on cardiorenal outcomes
Abstract
Background: To summarize the four recent sodium-glucose cotransporter 2 inhibitor (SGLT2i) trials: Dapagliflozin Effect on CardiovascuLAR Events (DECLARE-TIMI 58), CANagliflozin CardioVascular Assessment Study (CANVAS) Program, Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients-Removing Excess Glucose (EMPA-REG OUTCOME), Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE), and explore the potential determinants for their cardiovascular, renal, and safety outcomes.
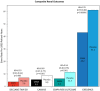
Results: The composite renal outcome event rates per 1000 patient-years for drug and placebo, as well as the corresponding relative risk reductions, were 3.7, 7.0, 47%; 5.5, 9.0, 40%; 6.3, 11.5, 46%; 43.2, 61.2, 30% for DECLARE-TIMI 58, CANVAS, EMPA-REG OUTCOME, and CREDENCE, respectively (event definitions varied across trials). The major adverse cardiovascular (CV) event rates per 1000 patient-years for drug and placebo, as well as the corresponding relative risk reductions, were 22.6, 24.2, 7%; 26.9, 31.5, 14%; 37.4, 43.9, 14%; 38.7, 48.7, 20% for DECLARE-TIMI 58, CANVAS, EMPA-REG OUTCOME, and CREDENCE, respectively. DECLARE-TIMI 58 had the fewest cardiorenal events and CREDENCE the most. These differences were presumably due to varying inclusion criteria resulting in DECLARE-TIMI 58 having the best baseline renal filtration function and CREDENCE the worst (mean estimated glomerular filtration rate 85.2, 76.5, 74, 56.2 mL/min/1.73 m2 for DECLARE-TIMI 58, CANVAS, EMPA-REG OUTCOME, and CREDENCE, respectively). Additionally, CREDENCE had considerably higher rates of albuminuria (median urinary albumin-creatinine ratios (UACR) were 927, 12.3, and 13.1 mg/g for CREDENCE, CANVAS, and DECLARE-TIMI 58, respectively; EMPA-REG OUTCOME had 59.4% UACR < 30, 28.6% UACR > 30-300, 11.0% UACR > 300 mg/g).
Conclusions: Dapagliflozin, empagliflozin, and canagliflozin have internally and externally consistent and biologically plausible class effects on cardiorenal outcomes. Baseline renal filtration function and degree of albuminuria are the most significant indicators of risk for both CV and renal events. Thus, these two factors also anticipate the greatest clinical benefit for SGLT2i.
Keywords: Albuminuria; CANVAS; CREDENCE; Canagliflozin; Cardiovascular death; Cardiovascular outcome trials; Chronic kidney disease; DECLARE-TIMI 58; Dapagliflozin; EMPA–REG OUTCOME; Empagliflozin; End-stage renal disease; Estimated glomerular filtration function; Heart failure hospitalization; Mortality; SGLT2 inhibitor.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2016 update: a report from the american heart association. Circulation. 2016;133:e38–e360. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

