Depletion of regulatory T cells increases T cell brain infiltration, reactive astrogliosis, and interferon-γ gene expression in acute experimental traumatic brain injury
- PMID: 31383034
- PMCID: PMC6683516
- DOI: 10.1186/s12974-019-1550-0
Depletion of regulatory T cells increases T cell brain infiltration, reactive astrogliosis, and interferon-γ gene expression in acute experimental traumatic brain injury
Erratum in
-
Correction to: Depletion of regulatory T cells increases T cell brain infiltration, reactive astrogliosis, and interferon-γ gene expression in acute experimental traumatic brain injury.J Neuroinflammation. 2019 Sep 7;16(1):176. doi: 10.1186/s12974-019-1577-2. J Neuroinflammation. 2019. PMID: 31493788 Free PMC article.
Abstract
Background: Traumatic brain injury (TBI) is a major cause of death and disability. T cells were shown to infiltrate the brain during the first days after injury and to exacerbate tissue damage. The objective of this study was to investigate the hitherto unresolved role of immunosuppressive, regulatory T cells (Tregs) in experimental TBI.
Methods: "Depletion of regulatory T cell" (DEREG) and wild type (WT) C57Bl/6 mice, treated with diphtheria toxin (DTx) to deplete Tregs or to serve as control, were subjected to the controlled cortical impact (CCI) model of TBI. Neurological and motor deficits were examined until 5 days post-injury (dpi). At the 5 dpi endpoint, (immuno-) histological, protein, and gene expression analyses were carried out to evaluate the consequences of Tregs depletion. Comparison of parametric or non-parametric data between two groups was done using Student's t test or the Mann-Whitney U test. For multiple comparisons, p values were calculated by one-way or two-way ANOVA followed by specific post hoc tests.
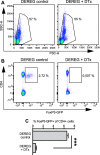
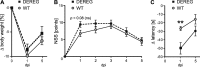
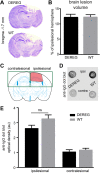
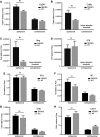
Results: The overall neurological outcome at 5 dpi was not different between DEREG and WT mice but more severe motor deficits occurred transiently at 1 dpi in DEREG mice. DEREG and WT mice did not differ in the extent of brain damage, blood-brain barrier (BBB) disruption, or neuronal excitotoxicity, as examined by lesion volumetry, immunoglobulin G (IgG) extravasation, or calpain-generated αII-spectrin breakdown products (SBDPs), respectively. In contrast, increased protein levels of glial fibrillary acidic protein (GFAP) and GFAP+ astrocytes in the ipsilesional brain tissue indicated exaggerated reactive astrogliosis in DEREG mice. T cell counts following anti-CD3 immunohistochemistry and gene expression analyses of Cd247 (CD3 subunit zeta) and Cd8a (CD8a) further indicated an increased number of T cells infiltrating the brain injury sites of DEREG mice compared to WT. These changes coincided with increased gene expression of pro-inflammatory interferon-γ (Ifng) in DEREG mice compared to WT in the injured brain.
Conclusions: The results show that the depletion of Tregs attenuates T cell brain infiltration, reactive astrogliosis, interferon-γ gene expression, and transiently motor deficits in murine acute traumatic brain injury.
Keywords: Astrocytes; Cytokines; Immune response; Inflammation; Microglia; T cells; Traumatic brain injury.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16(12):987–1048. - PubMed
-
- Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4–9. - PubMed
-
- Schäfer MK, Pfeiffer A, Jaeckel M, Pouya A, Dolga AM, Methner A. Regulators of mitochondrial Ca (2+) homeostasis in cerebral ischemia. Cell Tissue Res. 2014;357(2):395–405. - PubMed
-
- Toklu HZ, Tumer N. Oxidative stress, brain edema, blood-brain barrier permeability, and autonomic dysfunction from traumatic brain injury. 2015. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

