What the Clinical Microbiologist Should Know About Pharmacokinetics/Pharmacodynamics in the Era of Emerging Multidrug Resistance: Focusing on β-Lactam/β-Lactamase Inhibitor Combinations
- PMID: 31383269
- PMCID: PMC6686870
- DOI: 10.1016/j.cll.2019.05.006
What the Clinical Microbiologist Should Know About Pharmacokinetics/Pharmacodynamics in the Era of Emerging Multidrug Resistance: Focusing on β-Lactam/β-Lactamase Inhibitor Combinations
Abstract
As a class, β-lactamase inhibitors have proved successful in extending the clinical utility of β-lactam antibiotics by circumventing β-lactamase-mediated resistance. However, the rapid evolution of these β-lactamases calls for a critical reevaluation of the relationships between susceptibility, drug exposures, and bacterial response. The existing paradigm for in vitro susceptibility testing and development of β-lactam/β-lactamase inhibitor combinations may not optimally facilitate clinical use. Thus, alternative approaches for pairing these combinations and evaluating in vitro susceptibility are needed to provide better guidance to clinicians.
Keywords: Combination therapy; Gram-negative bacteria; Optimal dosing; Pharmacokinetics/pharmacodynamics; Susceptibility testing.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures
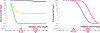

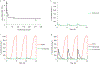
Similar articles
-
New β-lactam antibiotics and β-lactamase inhibitors.Expert Opin Ther Pat. 2010 Oct;20(10):1277-93. doi: 10.1517/13543776.2010.515588. Expert Opin Ther Pat. 2010. PMID: 20839927 Review.
-
New promising β-lactamase inhibitors for clinical use.Eur J Clin Microbiol Infect Dis. 2015 Jul;34(7):1303-8. doi: 10.1007/s10096-015-2375-0. Epub 2015 Apr 12. Eur J Clin Microbiol Infect Dis. 2015. PMID: 25864193 Review.
-
Three decades of beta-lactamase inhibitors.Clin Microbiol Rev. 2010 Jan;23(1):160-201. doi: 10.1128/CMR.00037-09. Clin Microbiol Rev. 2010. PMID: 20065329 Free PMC article. Review.
-
Progress toward inhibitors of metallo-β-lactamases.Future Med Chem. 2017 May;9(7):673-691. doi: 10.4155/fmc-2017-0007. Epub 2017 May 15. Future Med Chem. 2017. PMID: 28504895 Review.
-
Beta-lactam/beta-lactamase inhibitor combinations: pharmacodynamic considerations and possible role in the management of bacterial infections in the neutropenic host.J Antimicrob Chemother. 1998 Jun;41 Suppl D:43-9. doi: 10.1093/jac/41.suppl_4.43. J Antimicrob Chemother. 1998. PMID: 9688450 Review.
Cited by
-
New β-Lactam-β-Lactamase Inhibitor Combinations.Clin Microbiol Rev. 2020 Nov 11;34(1):e00115-20. doi: 10.1128/CMR.00115-20. Print 2020 Dec 16. Clin Microbiol Rev. 2020. PMID: 33177185 Free PMC article. Review.
-
Optimizing pharmacokinetics/pharmacodynamics of β-lactam/β-lactamase inhibitor combinations against high inocula of ESBL-producing bacteria.J Antimicrob Chemother. 2021 Jan 1;76(1):179-183. doi: 10.1093/jac/dkaa412. J Antimicrob Chemother. 2021. PMID: 33035321 Free PMC article.
-
Potential Inhibitory Effect of the Peptide Melittin Purified from Apis mellifera Venom on CTX-M-Type Extended-Spectrum β-Lactamases of Escherichia coli.Antibiotics (Basel). 2025 Apr 14;14(4):403. doi: 10.3390/antibiotics14040403. Antibiotics (Basel). 2025. PMID: 40298530 Free PMC article.
-
The latest advances in β-lactam/β-lactamase inhibitor combinations for the treatment of Gram-negative bacterial infections.Expert Opin Pharmacother. 2019 Dec;20(17):2169-2184. doi: 10.1080/14656566.2019.1660772. Epub 2019 Sep 9. Expert Opin Pharmacother. 2019. PMID: 31500471 Free PMC article. Review.
-
In vitro Optimization of Ceftazidime/Avibactam for KPC-Producing Klebsiella pneumoniae.Front Microbiol. 2021 Mar 4;12:618087. doi: 10.3389/fmicb.2021.618087. eCollection 2021. Front Microbiol. 2021. PMID: 33763041 Free PMC article.
References
-
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013 2014. Available at: https://www.cdc.gov/drugresistance/threat-report-2013/index.html. Accessed October 24, 2018.
-
- Cerceo E, Deitelzweig SB, Sherman BM, Amin AN. Multidrug-Resistant Gram-Negative Bacterial Infections in the Hospital Setting: Overview, Implications for Clinical Practice, and Emerging Treatment Options. Microb Drug Resist 2016;22(5):412–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

