Recombinant hemagglutinin produced from Chinese Hamster Ovary (CHO) stable cell clones and a PELC/CpG combination adjuvant for H7N9 subunit vaccine development
- PMID: 31383491
- PMCID: PMC7115541
- DOI: 10.1016/j.vaccine.2019.02.040
Recombinant hemagglutinin produced from Chinese Hamster Ovary (CHO) stable cell clones and a PELC/CpG combination adjuvant for H7N9 subunit vaccine development
Abstract
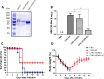
The novel H7N9 avian influenza A virus has caused human infections in China since 2013; some isolates from the fifth wave of infections have emerged as highly pathogenic avian influenza viruses. Recombinant hemagglutinin proteins of H7N9 viruses can be rapidly and efficiently produced with low-level biocontainment facilities. In this study, recombinant H7 antigen was obtained from engineered stable clones of Chinese Hamster Ovary (CHO) cells for subsequent large-scale production. The stable CHO cell clones were also adapted to grow in serum-free suspension cultures. To improve the immunogenicity of the recombinant H7 antigens, we evaluated the use of a novel combination adjuvant of PELC and CpG (PELC/CpG) to augment the anti-H7N9 immune responses in mice. We compared the effects with other adjuvants such as alum, AddaVax (MF59-like), and several Toll-like receptor ligands such as R848, CpG, and poly (I:C). With the PELC/CpG combination adjuvant, CHO cell-expressed rH7 antigens containing terminally sialylated complex type N-glycans were able to induce high titers of neutralizing antibodies in sera and conferred protection following live virus challenges. These data indicate that the CHO cell-expressed recombinant H7 antigens and a PELC/CpG combination adjuvant can be used for H7N9 subunit vaccine development.
Keywords: CHO cells; H7N9 vaccine; Hemagglutinin; PELC/CpG adjuvant.
Copyright © 2019 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

Similar articles
-
Recombinant hemagglutinin proteins formulated in a novel PELC/CpG adjuvant for H7N9 subunit vaccine development.Antiviral Res. 2017 Oct;146:213-220. doi: 10.1016/j.antiviral.2017.09.014. Epub 2017 Sep 22. Antiviral Res. 2017. PMID: 28947234
-
Squalene-adjuvanted H7N9 virus vaccine induces robust humoral immune response against H7N9 and H7N7 viruses.Vaccine. 2014 Jul 31;32(35):4485-4494. doi: 10.1016/j.vaccine.2014.06.043. Epub 2014 Jun 21. Vaccine. 2014. PMID: 24962757
-
Recombinant influenza A virus hemagglutinin HA2 subunit protects mice against influenza A(H7N9) virus infection.Arch Virol. 2015 Mar;160(3):777-86. doi: 10.1007/s00705-014-2314-x. Epub 2015 Jan 24. Arch Virol. 2015. PMID: 25616843
-
Cross-conservation of T-cell epitopes: now even more relevant to (H7N9) influenza vaccine design.Hum Vaccin Immunother. 2014;10(2):256-62. doi: 10.4161/hv.28135. Epub 2014 Feb 13. Hum Vaccin Immunother. 2014. PMID: 24525618 Free PMC article. Review.
-
CHO cells for virus-like particle and subunit vaccine manufacturing.Vaccine. 2024 Apr 11;42(10):2530-2542. doi: 10.1016/j.vaccine.2024.03.034. Epub 2024 Mar 19. Vaccine. 2024. PMID: 38503664 Review.
Cited by
-
A recombinant subunit vaccine candidate produced in plants elicits neutralizing antibodies against SARS-CoV-2 variants in macaques.Front Plant Sci. 2022 Sep 28;13:901978. doi: 10.3389/fpls.2022.901978. eCollection 2022. Front Plant Sci. 2022. PMID: 36247553 Free PMC article.
-
Effects of Adjuvants on the Immunogenicity and Efficacy of a Zika Virus Envelope Domain III Subunit Vaccine.Vaccines (Basel). 2019 Oct 27;7(4):161. doi: 10.3390/vaccines7040161. Vaccines (Basel). 2019. PMID: 31717890 Free PMC article.
-
Influenza H7N9 Virus Hemagglutinin with T169A Mutation Possesses Enhanced Thermostability and Provides Effective Immune Protection against Lethal H7N9 Virus Challenge in Chickens.Vaccines (Basel). 2023 Aug 2;11(8):1318. doi: 10.3390/vaccines11081318. Vaccines (Basel). 2023. PMID: 37631886 Free PMC article.
-
Supplementation of H7N9 Virus-Like Particle Vaccine With Recombinant Epitope Antigen Confers Full Protection Against Antigenically Divergent H7N9 Virus in Chickens.Front Immunol. 2022 Feb 21;13:785975. doi: 10.3389/fimmu.2022.785975. eCollection 2022. Front Immunol. 2022. PMID: 35265069 Free PMC article.
-
Immunogenic and Protective Properties of Recombinant Hemagglutinin of Influenza A (H5N8) Virus.Vaccines (Basel). 2024 Jan 29;12(2):143. doi: 10.3390/vaccines12020143. Vaccines (Basel). 2024. PMID: 38400127 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

