Bioavailability and cytosolic kinases modulate response to deoxynucleoside therapy in TK2 deficiency
- PMID: 31383553
- PMCID: PMC6710986
- DOI: 10.1016/j.ebiom.2019.07.037
Bioavailability and cytosolic kinases modulate response to deoxynucleoside therapy in TK2 deficiency
Abstract
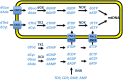
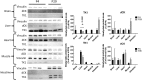
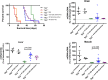
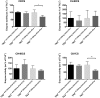
Background: TK2 is a nuclear gene encoding the mitochondrial matrix protein thymidine kinase 2 (TK2), a critical enzyme in the mitochondrial nucleotide salvage pathway. Deficiency of TK2 activity causes mitochondrial DNA (mtDNA) depletion, which in humans manifests predominantly as a mitochondrial myopathy with onset typically in infancy and childhood. We previously showed that oral treatment of the Tk2 H126N knock-in mouse model (Tk2-/-) with the TK2 substrates, deoxycytidine (dCtd) and thymidine (dThd), delayed disease onset and prolonged median survival by 3-fold. Nevertheless, dCtd + dThd treated Tk2-/- mice showed mtDNA depletion in brain as early as postnatal day 13 and in virtually all other tissues at age 29 days.
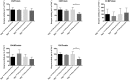
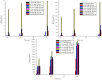
Methods: To enhance mechanistic understanding and efficacy of dCtd + dThd therapy, we studied the bioavailability of dCtd and dThd in various tissues as well as levels of the cytosolic enzymes, TK1 and dCK that convert the deoxynucleosides into dCMP and dTMP.
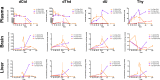
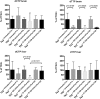
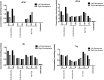
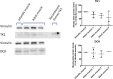
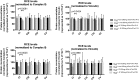
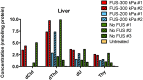
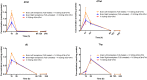
Findings: Parenteral treatment relative to oral treatment produced higher levels of dCtd and dThd and improved mtDNA levels in liver and heart, but did not ameliorate molecular defects in brain or prolong survival. Down-regulation of TK1 correlated with temporal- and tissue-specificity of response to dCtd + dThd. Finally, we observed in human infant and adult muscle expression of TK1 and dCK, which account for the long-term efficacy to dCtd + dThd therapy in TK2 deficient patients.
Interpretations: These data indicate that the cytosolic pyrimidine salvage pathway enzymes TK1 and dCK are critical for therapeutic efficacy of deoxynucleoside therapy for Tk2 deficiency. FUND: National Institutes of Health P01HD32062.
Keywords: Deoxycytidine; Nucleoside; TK2; Thymidine; Thymidine kinase 2; mtDNA depletion syndrome.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
MH is a co-inventor on a patent application for deoxynucleoside therapies for mitochondrial DNA depletion syndrome including TK2 deficiency. Columbia University Irving Medical Center (CUIMC) has licensed the pending patent applications related to the technology to Modis Therapeutics, Inc. (formerly Meves Pharmaceuticals, Inc.) and CUIMC may be eligible to receive payments related to the development and commercialization of the technology. MH is a paid consultant to Modis Therapeutics, Inc. This relationship is de minimus for Columbia University Medical Center (MH). The other authors declare no conflicts of interest.
Figures


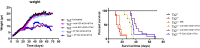
Comment in
-
Insights into deoxyribonucleoside therapy for mitochondrial TK2 deficient mtDNA depletion.EBioMedicine. 2019 Sep;47:14-15. doi: 10.1016/j.ebiom.2019.08.005. Epub 2019 Aug 8. EBioMedicine. 2019. PMID: 31402231 Free PMC article. No abstract available.
References
-
- Wang L. Mitochondrial purine and pyrimidine metabolism and beyond. Nucleosides Nucleotides Nucleic Acids. 2016;35(10−12):578–594. - PubMed
-
- Saada A., Shaag A., Mandel H., Nevo Y., Eriksson S., Elpeleg O. Mutant mitochondrial thymidine kinase in mitochondrial DNA depletion myopathy. Nat Genet. 2001;29(3):342–344. - PubMed
-
- Saada A., Ben-Shalom E., Zyslin R., Miller C., Mandel H., Elpeleg O. Mitochondrial deoxyribonucleoside triphosphate pools in thymidine kinase 2 deficiency. Biochem Biophys Res Commun. 2003;310(3):963–966. - PubMed
-
- Behin A., Jardel C., Claeys K.G. Adult cases of mitochondrial DNA depletion due to TK2 defect: an expanding spectrum. Neurology. 2012;78(9):644–648. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

