Restricted myeloperoxidase epitopes drive the adaptive immune response in MPO-ANCA vasculitis
- PMID: 31383567
- PMCID: PMC6930338
- DOI: 10.1016/j.jaut.2019.102306
Restricted myeloperoxidase epitopes drive the adaptive immune response in MPO-ANCA vasculitis
Abstract
Background: Treatment of autoimmune diseases has relied on broad immunosuppression. Knowledge of specific interactions between human leukocyte antigen (HLA), the autoantigen, and effector immune cells, provides the foundation for antigen-specific therapies. These studies investigated the role of HLA, specific myeloperoxidase (MPO) epitopes, CD4+ T cells, and ANCA specificity in shaping the immune response in patients with anti-neutrophil cytoplasmic autoantibody (ANCA) vasculitis.
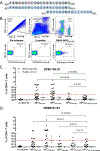
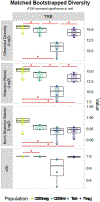
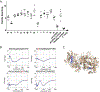
Methods: HLA sequence-based typing identified enriched alleles in our patient population (HLA-DPB1*04:01 and HLA-DRB4*01:01), while in silico and in vitro binding studies confirmed binding between HLA and specific MPO epitopes. Class II tetramers with MPO peptides were utilized to detect autoreactive CD4+ T cells. TCR sequencing was performed to determine the clonality of T cell populations. Longitudinal peptide ELISAs assessed the temporal nature of anti-MPO447-461 antibodies. Solvent accessibility combined with chemical modification determined the buried regions of MPO.
Results: We identified a restricted region of MPO that was recognized by both CD4+ T cells and ANCA. The autoreactive T cell population contained CD4+CD25intermediateCD45RO+ memory T cells and secreted IL-17A. T cell receptor (TCR) sequencing demonstrated that autoreactive CD4+ T cells had significantly less TCR diversity when compared to naïve and memory T cells, indicating clonal expansion. The anti-MPO447-461 autoantibody response was detectable at onset of disease in some patients and correlated with disease activity in others. This region of MPO that is targeted by both T cells and antibodies is not accessible to solvent or chemical modification, indicating these epitopes are buried.
Conclusions: These observations reveal interactions between restricted MPO epitopes and the adaptive immune system within ANCA vasculitis that may inform new antigen-specific therapies in autoimmune disease while providing insight into immunopathogenesis.
Keywords: ANCA specificity; ANCA vasculitis; Autoreactive T cells; Epitope specificity; Immunodominant epitopes.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures




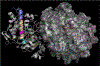
References
-
- Falk RJ, and Jennette JC 2002. ANCA are pathogenic--oh yes they are! J Am Soc Nephrol 13:1977–1979. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

