Longer-term efficiency and safety of increasing the frequency of whole blood donation (INTERVAL): extension study of a randomised trial of 20 757 blood donors
- PMID: 31383583
- PMCID: PMC7029279
- DOI: 10.1016/S2352-3026(19)30106-1
Longer-term efficiency and safety of increasing the frequency of whole blood donation (INTERVAL): extension study of a randomised trial of 20 757 blood donors
Abstract
Background: The INTERVAL trial showed that, over a 2-year period, inter-donation intervals for whole blood donation can be safely reduced to meet blood shortages. We extended the INTERVAL trial for a further 2 years to evaluate the longer-term risks and benefits of varying inter-donation intervals, and to compare routine versus more intensive reminders to help donors keep appointments.
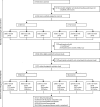
Methods: The INTERVAL trial was a parallel group, pragmatic, randomised trial that recruited blood donors aged 18 years or older from 25 static donor centres of NHS Blood and Transplant across England, UK. Here we report on the prespecified analyses after 4 years of follow-up. Participants were whole blood donors who agreed to continue trial participation on their originally allocated inter-donation intervals (men: 12, 10, and 8 weeks; women: 16, 14, and 12 weeks). They were further block-randomised (1:1) to routine versus more intensive reminders using computer-generated random sequences. The prespecified primary outcome was units of blood collected per year analysed in the intention-to-treat population. Secondary outcomes related to safety were quality of life, self-reported symptoms potentially related to donation, haemoglobin and ferritin concentrations, and deferrals because of low haemoglobin and other factors. This trial is registered with ISRCTN, number ISRCTN24760606, and has completed.
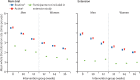
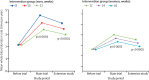
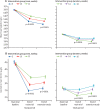
Findings: Between Oct 19, 2014, and May 3, 2016, 20 757 of the 38 035 invited blood donors (10 843 [58%] men, 9914 [51%] women) participated in the extension study. 10 378 (50%) were randomly assigned to routine reminders and 10 379 (50%) were randomly assigned to more intensive reminders. Median follow-up was 1·1 years (IQR 0·7-1·3). Compared with routine reminders, more intensive reminders increased blood collection by a mean of 0·11 units per year (95% CI 0·04-0·17; p=0·0003) in men and 0·06 units per year (0·01-0·11; p=0·0094) in women. During the extension study, each week shorter inter-donation interval increased blood collection by a mean of 0·23 units per year (0·21-0·25) in men and 0·14 units per year (0·12-0·15) in women (both p<0·0001). More frequent donation resulted in more deferrals for low haemoglobin (odds ratio per week shorter inter-donation interval 1·19 [95% CI 1·15-1·22] in men and 1·10 [1·06-1·14] in women), and lower mean haemoglobin (difference per week shorter inter-donation interval -0·84 g/L [95% CI -0·99 to -0·70] in men and -0·45 g/L [-0·59 to -0·31] in women) and ferritin concentrations (percentage difference per week shorter inter-donation interval -6·5% [95% CI -7·6 to -5·5] in men and -5·3% [-6·5 to -4·2] in women; all p<0·0001). No differences were observed in quality of life, serious adverse events, or self-reported symptoms (p>0.0001 for tests of linear trend by inter-donation intervals) other than a higher reported frequency of doctor-diagnosed low iron concentrations and prescription of iron supplements in men (p<0·0001).
Interpretation: During a period of up to 4 years, shorter inter-donation intervals and more intensive reminders resulted in more blood being collected without a detectable effect on donors' mental and physical wellbeing. However, donors had decreased haemoglobin concentrations and more self-reported symptoms compared with the initial 2 years of the trial. Our findings suggest that blood collection services could safely use shorter donation intervals and more intensive reminders to meet shortages, for donors who maintain adequate haemoglobin concentrations and iron stores.
Funding: NHS Blood and Transplant, UK National Institute for Health Research, UK Medical Research Council, and British Heart Foundation.
Copyright © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Williamson LM, Devine DV. Challenges in the management of the blood supply. Lancet. 2013;381:1866–1875. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

