Incorporation of a chiral gem-disubstituted nitrogen heterocycle yields an oxazolidinone antibiotic with reduced mitochondrial toxicity
- PMID: 31383589
- PMCID: PMC6711789
- DOI: 10.1016/j.bmcl.2019.07.024
Incorporation of a chiral gem-disubstituted nitrogen heterocycle yields an oxazolidinone antibiotic with reduced mitochondrial toxicity
Abstract
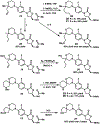
gem-Disubstituted N-heterocycles are rarely found in drugs, despite their potential to improve the drug-like properties of small molecule pharmaceuticals. Linezolid, a morpholine heterocycle-containing oxazolidinone antibiotic, exhibits significant side effects associated with human mitochondrial protein synthesis inhibition. We synthesized a gem-disubstituted linezolid analogue that when compared to linezolid, maintains comparable (albeit slightly diminished) activity against bacteria, comparable in vitro physicochemical properties, and a decrease in undesired mitochondrial protein synthesis (MPS) inhibition. This research contributes to the structure-activity-relationship data surrounding oxazolidinone MPS inhibition, and may inspire investigations into the utility of gem-disubstituted N-heterocycles in medicinal chemistry.
Keywords: Allylic alkylation; Antibiotic; Heterocycle; Linezolid; Mitochondria.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures
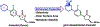

References
-
- Vitaku E; Smith DT; Njardarson JT Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. - PubMed
-
- Lovering F; Bikker J; Humblet C Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756. - PubMed
-
- Lovering F Escape from Flatland 2: Complexity and Promiscuity. Med. Chem. Commun. 2013, 4, 515–519.
-
- Ippolito JA; Kanyo ZF; Wang D; Franceschi FJ; Moore PB; Steitz TA; Duffy EM Crystal Structure of the Oxazolidinone Antibiotic Linezolid Bound to the 50S Ribosomal Subunit. J. Med. Chem. 2008, 51, 3353–3356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Medical
Research Materials

