Mitochondrial plasticity in cell fate regulation
- PMID: 31383739
- PMCID: PMC6755789
- DOI: 10.1074/jbc.REV118.000828
Mitochondrial plasticity in cell fate regulation
Abstract
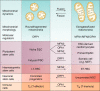
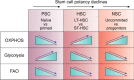
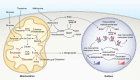
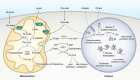
Mitochondria are considered highly plastic organelles. This plasticity enables the mitochondria to undergo morphological and functional changes in response to cellular demands. Stem cells also need to remain functionally plastic (i.e. to have the ability to "decide" whether to remain quiescent or to undergo activation upon signaling cues to support tissue function and homeostasis). Mitochondrial plasticity is thought to enable this reshaping of stem cell functions, integrating signaling cues with stem cell outcomes. Indeed, recent evidence highlights the crucial role of maintaining mitochondrial plasticity for stem cell biology. For example, tricarboxylic acid (TCA) cycle metabolites generated and metabolized in the mitochondria serve as cofactors for epigenetic enzymes, thereby coupling mitochondrial metabolism and transcriptional regulation. Another layer of mitochondrial plasticity has emerged, pointing toward mitochondrial dynamics in regulating stem cell fate decisions. Imposing imbalanced mitochondrial dynamics by manipulating the expression levels of the key molecular regulators of this process influences cellular outcomes by changing the nuclear transcriptional program. Moreover, reactive oxygen species have also been shown to play an important role in regulating transcriptional profiles in stem cells. In this review, we focus on recent findings demonstrating that mitochondria are essential regulators of stem cell activation and fate decisions. We also discuss the suggested mechanisms and alternative routes for mitochondria-to-nucleus communications.
Keywords: ROS signaling; cell signaling; epigenetics; histone modification; metabolic cross-talk; metabolism; mitochondria; mitochondrial DNA (mtDNA); mitochondrial metabolism; molecular dynamics; nucleus; self-renewal; stem cells.
© 2019 Bahat and Gross.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures
References
-
- Maryanovich M., Zahalka A. H., Pierce H., Pinho S., Nakahara F., Asada N., Wei Q., Wang X., Ciero P., Xu J., Leftin A., and Frenette P. S. (2018) Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat. Med. 24, 782–791 10.1038/s41591-018-0030-x - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

