Characterization of subcutaneous and omental adipose tissue in patients with obesity and with different degrees of glucose impairment
- PMID: 31383894
- PMCID: PMC6683173
- DOI: 10.1038/s41598-019-47719-y
Characterization of subcutaneous and omental adipose tissue in patients with obesity and with different degrees of glucose impairment
Abstract
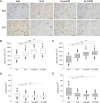
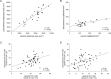
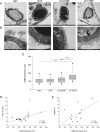
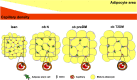
Although obesity represents a risk factor for the development of type 2 diabetes mellitus (T2DM), the link between these pathological conditions is not so clear. The manner in which the different elements of adipose tissue (AT) interplay in order to grow has been suggested to have a role in the genesis of metabolic complications, but this has not yet been fully addressed in humans. Through IHC, transmission electron microscopy, cytometry, and in vitro cultures, we described the morphological and functional changes of subcutaneous and visceral AT (SAT and VAT) in normoglycemic, prediabetic and T2DM patients with obesity compared to lean subjects. In both SAT and VAT we measured a hypertrophic and hyperplastic expansion, causing similar vascular rarefaction in obese patients with different degrees of metabolic complications. Capillaries display dysfunctional basement membrane thickening only in T2DM patients evidencing VAT as a new target of T2DM microangiopathy. The largest increase in adipocyte size and decrease in adipose stem cell number and adipogenic potential occur both in T2DM and in prediabetes. We showed that SAT and VAT remodeling with stemness deficit is associated with early glucose metabolism impairment suggesting the benefit of an AT-target therapy controlling hypertrophy and hyperplasia already in prediabetic obese patients.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

