Red blood cell membrane-camouflaged nanoparticles: a novel drug delivery system for antitumor application
- PMID: 31384529
- PMCID: PMC6663920
- DOI: 10.1016/j.apsb.2019.01.011
Red blood cell membrane-camouflaged nanoparticles: a novel drug delivery system for antitumor application
Abstract
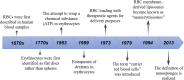
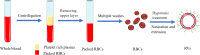
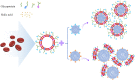
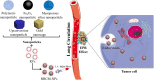
Erythrocytes (red blood cells, RBCs) are the most abundant circulating cells in the blood and have been widely used in drug delivery systems (DDS) because of their features of biocompatibility, biodegradability, and long circulating half-life. Accordingly, a "camouflage" comprised of erythrocyte membranes renders nanoparticles as a platform that combines the advantages of native erythrocyte membranes with those of nanomaterials. Following injection into the blood of animal models, the coated nanoparticles imitate RBCs and interact with the surroundings to achieve long-term circulation. In this review, the biomimetic platform of erythrocyte membrane-coated nano-cores is described with regard to various aspects, with particular focus placed on the coating mechanism, preparation methods, verification methods, and the latest anti-tumor applications. Finally, further functional modifications of the erythrocyte membranes and attempts to fuse the surface properties of multiple cell membranes are discussed, providing a foundation to stimulate extensive research into multifunctional nano-biomimetic systems.
Keywords: ABC, accelerated blood clearance; APCs, antigen presenting cells; Antitumor; AuNCs, gold nanocages; AuNPs, gold nanoparticles; Biomimetic nanoparticles; C8bp, C8 binding protein; CR1, complement receptor 1; DAF, decay accelerating factor; DDS, drug delivery systems; DLS, dynamic light scattering; Dox, doxorubicin; Drug delivery; ECM, extracellular matrix; EPR, enhanced permeability and retention; ETA, endothelin A; EpCam, epithelial cell adhesion molecule; FA, folic acid; GA, gambogic acid; H&E, hematoxylin and eosin; HRP, homologous restriction protein; MCP, membrane cofactor protein; MNCs, magnetic nanoclusters; MNs, magnetic nanoparticles; MPS, mononuclear phagocyte system; MRI, magnetic resonance imaging; MSNs, mesoporous silica nanoparticles; Membrane; NIR, near-infrared radiation; Nanoparticles; PAI, photoacoustic imaging; PBS, phosphate buffered saline; PCL, poly(caprolactone); PDT, photodynamic therapy; PEG, polyethylene glycol; PFCs, perfluorocarbons; PLA, poly(lactide acid); PLGA, poly(d,l-lactide-co-glycolide); PPy, polypyrrole; PS, photosensitizers; PTT, photothermal therapy; PTX, paclitaxel; RBCM-NPs, RBCM-coated nanoparticles; RBCMs, RBC membranes; RBCs, red blood cells; RES, reticuloendothelial system; ROS, reactive oxygen species; RVs, RBCM-derived vesicles; Red blood cells; SEM, scanning electron microscopy; SIRPα, signal-regulatory protein alpha; TEM, transmission electron microscopy; TEMPO, 2,2,6,6-tetramethylpiperidin-1-yl oxyl; TPP, triphenylphosphonium; UCNPs, upconversion nanoparticles; UV, ultraviolet; rHuPH20, recombinant hyaluronidase, PH20.
Figures


References
-
- Iwamoto T. Clinical application of drug delivery systems in cancer chemotherapy: review of the efficacy and side effects of approved drugs. Biol Pharm Bull. 2013;36:715–718. - PubMed
-
- Chen M.C., Lin Z.W., Ling M.H. Near-infrared light-activatable microneedle system for treating superficial tumors by combination of chemotherapy and photothermal therapy. ACS Nano. 2016;10:93–101. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

