Signals from the various immune cells in promoting food allergy-induced eosinophilic esophagitis like disease
- PMID: 31384583
- PMCID: PMC6676061
- DOI: 10.5415/apallergy.2019.9.e28
Signals from the various immune cells in promoting food allergy-induced eosinophilic esophagitis like disease
Abstract
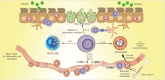
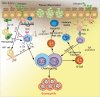
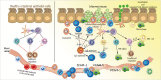
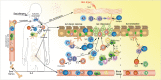
Eosinophilic esophagitis (EoE) is a recently recognized esophageal inflammatory disease with clinical manifestations arising from esophageal dysfunction. The etiology of EoE is currently being clarified and food allergy is evolving as the central cornerstone of EoE disease pathogenesis. Given the large number of eosinophils in the esophagus of people with EoE verified by data from murine models EoE is widely considered as the hallmark T-helper type 2 (Th2) disease of the esophagus. It is also known that some eosinophilic inflammation is controlled by other subsets of T cells such as Th9 or Th17 and control is also exerted by type 2 innate lymphoid cells acting together with basophils. In this paper we review results from molecular studies of mouse models in light of the results from the first clinical trials targeting key cytokines in humans and present in-depth molecular understanding of EoE.
Keywords: Elimination diet; Eosinophilic esophagitis; Food allergy; Pathogenesis; Therapy.
Figures
References
-
- Abonia JP, Rothenberg ME. Eosinophilic esophagitis: rapidly advancing insights. Annu Rev Med. 2012;63:421–434. - PubMed
-
- Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, Burks AW, Chehade M, Collins MH, Dellon ES, Dohil R, Falk GW, Gonsalves N, Gupta SK, Katzka DA, Lucendo AJ, Markowitz JE, Noel RJ, Odze RD, Putnam PE, Richter JE, Romero Y, Ruchelli E, Sampson HA, Schoepfer A, Shaheen NJ, Sicherer SH, Spechler S, Spergel JM, Straumann A, Wershil BK, Rothenberg ME, Aceves SS. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20. - PubMed
-
- Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology. 1995;109:1503–1512. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
