Risk factors for modified vaccine effectiveness of the live attenuated zoster vaccine among the elderly in England
- PMID: 31384729
- PMCID: PMC6668231
- DOI: 10.1016/j.jvacx.2019.100007
Risk factors for modified vaccine effectiveness of the live attenuated zoster vaccine among the elderly in England
Abstract
Background: The United Kingdom introduced routine vaccination with the live-attenuated zoster vaccine for 70 year-olds in 2013, with the vaccine also offered to 79 year-olds as part of a catch-up campaign. In the subsequent years, the catch-up campaign was extended to also include adults aged 78 years. We investigated 14 pre-identified potential risk factors for potential modified vaccine effectiveness.
Methods: This retrospective cohort study in England included subjects born in 1943-1946 (the routine cohort) and in 1934-1937 (the catch-up cohort). We used the Clinical Practice Research Datalink (CPRD) to identify herpes zoster (HZ) cases and the risk factors: age, gender, ethnicity, socio-economic status, asthma, type 2 diabetes, chronic obstructive pulmonary disease, smoking, body mass index, immunosuppression, history of HZ, co-administration with influenza or pneumococcal vaccine. We derived HZ incidence by risk groups, overall vaccine effectiveness (VE) and modified VE expressed as relative differences in VE from Poisson regression models.
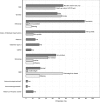
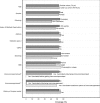
Results: Overall VE was 66.8% [95% CI: 62.2; 71.0]. Two out of the 14 investigated risk factors modified the HZ VE. Notably, lower VE was observed in diabetics and in persons with a history of HZ with relative differences in VE of -22·2%, [95% CI: -39·6, -4·5] and -22·5%, [95% CI: -44·9, -0·1].
Conclusions: Live-attenuated zoster vaccine protection against HZ was lower in type 2 diabetics and in subjects with a history of HZ. Contrary to clinical trial results, age did not affect the observed VE. Further study is required to gain insights into why certain risk groups are less protected. Identifying and understanding the effect modifiers of VE is important for future vaccine development as well as vaccine recommendations.
Keywords: Effectiveness; Herpes zoster; Shingles; Vaccine; Vaccine failure.
Figures

References
-
- Gauthier A., Breuer J., Carrington D., Martin M., Remy V. Epidemiology and cost of herpes zoster and post-herpetic neuralgia in the United Kingdom. Epidemiol Infect. 2009;137:38–47. - PubMed
-
- European Medicines Agency. Zostavax (shingles [herpes zoster]) vaccine [live]): summary of product characteristics. Available from: <http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info...>.
-
- Oxman M.N., Levin M.J., Johnson G.R., Schmader K.E., Straus S.E., Gelb L.D. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. - PubMed
LinkOut - more resources
Full Text Sources

