Sex hormone intake in female blood donors: impact on haemolysis during cold storage and regulation of erythrocyte calcium influx by progesterone
- PMID: 31385799
- PMCID: PMC6683868
- DOI: 10.2450/2019.0053-19
Sex hormone intake in female blood donors: impact on haemolysis during cold storage and regulation of erythrocyte calcium influx by progesterone
Abstract
Background: Sex hormone intake in blood donors may affect the quality of red blood cell (RBC) products via modulation of RBC function and predisposition to haemolysis during cold storage. The aims of this study were to evaluate the association between female sex hormone intake and RBC storage outcomes, and to examine possible mechanisms by which sex hormones interact with RBCs.
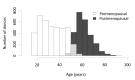
Materials and methods: Sex hormone intake by race/ethnicity and menopausal status, and association analyses between hormone intake and donor scores of storage, osmotic or oxidative haemolysis, were evaluated in 6,636 female donors who participated in the National Heart, Lung and Blood Institute's RBC-Omics study. A calcium fluorophore, Fluo-3AM, was used to define RBC calcium influx in response to exogenous sex hormones or transient receptor potential cation (TRPC) channel drugs.
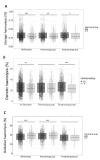
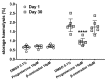
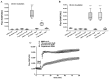
Results: Sex hormone intake was more prevalent in premenopausal women from all racial groups (18-31%) than in postmenopausal women (4-8%). Hormone intake was significantly (p<0.0001) associated with reduced storage haemolysis in all females, reduced osmotic haemolysis in postmenopausal donors (23.1±10.2% vs 26.8±12.0% in controls, p<0.001), and enhanced susceptibility to oxidative haemolysis in premenopausal women. In vitro, supraphysiological levels of progesterone (10 μmol/L), but not 17β-oestradiol or testosterone, inhibited calcium influx into RBC and was associated with lower spontaneous haemolysis after 30 days of cold storage (0.95±0.18% vs 1.85±0.35% in controls, p<0.0001) or in response to a TRPC6 activator.
Conclusions: Sex hormone intake in female donors is associated with changes in RBC predisposition to haemolysis. Menstrual status and the type of hormone preparation may contribute to differences in haemolytic responses of female RBCs to osmotic and oxidative stress. Progesterone modulates calcium influx into RBC via a mechanism that may involve interactions with membrane TRPC6 channels.
Conflict of interest statement
The Authors declare no conflicts of interest.
Figures


References
-
- Daniels K, Daugherty J, Jones J, Mosher W. Current contraceptive use and variation by selected characteristics among women aged 15–44: United States, 2011–2013. Natl Health Stat Report. 2015:1–14. - PubMed
-
- De Leo V, Musacchio MC, Cappelli V, et al. Hormonal contraceptives: pharmacology tailored to women’s health. Hum Reprod Update. 2016;22:634–46. - PubMed
-
- Nelson HD, Humphrey LL, Nygren P, et al. Postmenopausal hormone replacement therapy scientific review. JAMA. 2002;288:872–81. - PubMed
MeSH terms
Substances
Grants and funding
- HHSN268201100008C/HL/NHLBI NIH HHS/United States
- HHSN268201100007C/HL/NHLBI NIH HHS/United States
- HHSN268201100005I/HL/NHLBI NIH HHS/United States
- HHSN268201100002C/WH/WHI NIH HHS/United States
- HHSN268201100002I/HL/NHLBI NIH HHS/United States
- HHSN268201100001C/WH/WHI NIH HHS/United States
- HHSN268201100004C/WH/WHI NIH HHS/United States
- HHSN268201100001I/HL/NHLBI NIH HHS/United States
- HHSN268201100009I/HL/NHLBI NIH HHS/United States
- HHSN268201100005G/HL/NHLBI NIH HHS/United States
- HHSN268201100004I/HL/NHLBI NIH HHS/United States
- HHSN268201100008I/HL/NHLBI NIH HHS/United States
- R01 HL134653/HL/NHLBI NIH HHS/United States
- HHSN268201100003C/WH/WHI NIH HHS/United States
- HHSN268201100006C/HL/NHLBI NIH HHS/United States
- R01 HL098032/HL/NHLBI NIH HHS/United States
- HHSN268201100009C/HL/NHLBI NIH HHS/United States
- HHSN268201100005C/HL/NHLBI NIH HHS/United States
- HHSN268201100007I/HL/NHLBI NIH HHS/United States
- HHSN268201100003I/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
