Donor Iron Deficiency Study (DIDS): protocol of a study to test whether iron deficiency in blood donors affects red blood cell recovery after transfusion
- PMID: 31385800
- PMCID: PMC6683873
- DOI: 10.2450/2019.0066-19
Donor Iron Deficiency Study (DIDS): protocol of a study to test whether iron deficiency in blood donors affects red blood cell recovery after transfusion
Abstract
Background: Despite fulfilling all requirements for blood donation, a large proportion of regular blood donors are iron deficient. Red blood cells (RBC) from iron-deficient donors may be particularly susceptible to damage induced by standard refrigerated storage. Herein, we present a study protocol for testing whether correcting iron deficiency in donors with iron-deficient erythropoiesis will improve the quality of their refrigerator-stored RBC.
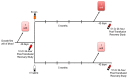
Materials and methods: This is a randomised, controlled, double-blind clinical trial. Sixty healthy regular donors who meet donation standards, while exhibiting iron-deficient erythropoiesis by laboratory testing criteria, will donate a single standard RBC unit that will be leucoreduced and stored in a refrigerator under standard conditions for 40-42 days. A 51Cr-radiolabelled 24-hour RBC recovery study will be performed and then these donors will be randomised to receive, in a double-blinded fashion, either intravenous saline, as a control, or low-molecular weight iron dextran (1 g), to provide total iron repletion. Four to six months later, they will donate a second RBC unit, which will be similarly stored, and autologous 51Cr-labelled 24-hour post-transfusion RBC recovery will again be determined.
Results: The primary endpoint will be the change in 24-hour post-transfusion recovery from the first to the second donation. The primary outcome will be the group mean difference in the primary endpoints between the group receiving intravenous saline and the group receiving intravenous iron dextran. Secondary outcomes will be quality of life, fatigue, and emotional health, assessed by surveys.
Conclusion: This study will provide definitive evidence as to whether donor iron deficiency affects the quality of the blood supply and will assess the severity of symptoms affecting iron-deficient blood donors.
Conflict of interest statement
SLS is on the scientific advisory board of Hemanext, Inc. and is a consultant for Tioma, Inc. The other Authors declare that they have no competing financial interests.
Figures
References
-
- Whitaker B. The 2009 National Blood Collection and Utilization Survey Report. Washington, DC: US Department of Health and Human Services, Office of the Assistant Secretary for Health; 2011.
-
- Goldman M, Uzicanin S, Scalia V, O’Brien SF. Iron deficiency in Canadian blood donors. Transfusion. 2013;54:775–9. - PubMed
-
- Smith GA, Fisher SA, Dorée C, Roberts DJ. A systematic review of factors associated with the deferral of donors failing to meet low haemoglobin thresholds. Transfus Med. 2013;23:309–20. - PubMed
-
- Birgegard G, Schneider K, Ulfberg J. High incidence of iron depletion and restless leg syndrome (RLS) in regular blood donors: intravenous iron sucrose substitution more effective than oral iron. Vox Sang. 2010;99:354–61. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
