Phase I cancer clinical trials
- PMID: 31385964
- PMCID: PMC6655360
- DOI: 10.1093/nop/npw014
Phase I cancer clinical trials
Abstract
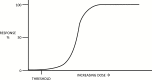
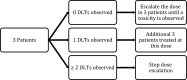
An efficient phase I trial is a crucial step in developing a new drug in a safe and timely manner. The main objective of a phase I trial is to determine the maximum tolerated dose in order to recommend the dose for a phase II trial. There are many designs that are implemented in phase I trials. Rule-based designs such as the traditional 3 + 3 method and rolling six design are easy to implement and assess for safety using a conservative approach. Model-based designs such as the continual reassessment method and the time-to-event continual reassessment method use mathematical models to increase the precision of dose estimation. The advantages and shortcomings of these designs, along with other designs, are reviewed.
Keywords: clinical trials; phase I; trial design..
Figures
References
-
- Storer BE. Design and analysis of phase I clinical trials. Biometrics. 1989;925–937. - PubMed
-
- Korn EL, Midthune D, Chen TT, Rubinstein LV, Christian MC, Simon RM. A comparison of two phase I trial designs. Stat Med. 1994;13(18):1799–1806. - PubMed
-
- Rogatko A, Schoeneck D, Jonas W, Tighiouart M, Khuri FR, Porter A. Translation of innovative designs into phase I trials. J Clin Oncol. 2007;25(31):4982–4986. - PubMed
-
- Arbuck SG. Workshop on phase I study design Ninth NCI/EORTC New Drug Development Symposium, Amsterdam. Ann Oncol. 1996;7(6):567–573. - PubMed

